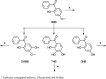
Simultaneous Quantitation of 2-Hydroxy-4-Methoxybenzophenone, a Sunscreen Ingredient, and its Metabolites in Harlan Sprague Dawley Rat Plasma Following Perinatal Dietary Exposure
- PMID: 28977387
- PMCID: PMC5890876
- DOI: 10.1093/jat/bkx070
Simultaneous Quantitation of 2-Hydroxy-4-Methoxybenzophenone, a Sunscreen Ingredient, and its Metabolites in Harlan Sprague Dawley Rat Plasma Following Perinatal Dietary Exposure
Abstract
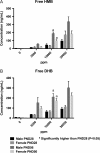
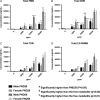
2-Hydroxy-4-methoxybenzophenone (HMB) is a common ingredient in sunscreens and other personal care products and thus significant potential exists for human exposure. HMB was nominated to the National Toxicology Program (NTP) for testing due to its high exposure through consumer products and inadequate toxicological data at the time, which also included increasing concern for the potential effects of HMB on reproduction and development. HMB is metabolized to numerous metabolites in vivo and in vitro including 2,4-dihydroxybenzophenone (DHB), 2,3,4-trihydroxybenzophenone (THB) and 2,5-dihydroxy-4-methoxybenzophenone (2,5-DHMB) as well as their corresponding glucuronide and/or sulfate conjugates. In this study, we have developed and validated a liquid chromatography-tandem mass spectrometry method to quantitate free (unconjugated) HMB and DHB, and total (combined conjugated and unconjugated) HMB, DHB, THB and 2,5-DHMB. The method was successfully applied to quantitate these analytes in plasma from postnatal day 28 and 56 male and female Harlan Sprague Dawley rat pups following perinatal dietary exposure to 0 (control), 3,000, 10,000 and 30,000 ppm HMB beginning on gestational Day 6. All determined analyte concentrations increased with increasing dose and were significantly higher than the controls at both timepoints. All the total analytes were quantified in all plasma samples and total concentrations were considerably higher than free, suggesting extensive conjugation. Mean concentrations of total HMB and DHB were higher (~100-300-fold) than the free HMB and DHB concentrations, and total concentrations in plasma were approximately HMB≈DHB > 2,5-DHMB»THB. Free and total analyte plasma concentrations were not sex-dependent and in general, both free and total analytes were detected in the control samples. Comparison of our rat data, using the internal dose, with human data available in the literature suggests that the rat doses used in our studies were within 4-fold of the human dose.
Published by Oxford University Press 2017.
Figures

References
-
- Database, E.s.S.D.C. Environmental Working Group Skin Deep Cosmetic Database. 2016; http://www.ewg.org/skindeep/search.php?query=HMB&h=Search.
-
- Liao C., Kannan K. (2014) Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: an assessment of human exposure. Environmental Science & Technology, 48, 4103–4109. - PubMed
-
- Gonzalez H., Farbrot A., Larko O., Wennberg A.M. (2006) Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. The British Journal of Dermatology, 154, 337–340. - PubMed
-
- Sarveiya V., Risk S., Benson H.A. (2004) Liquid chromatographic assay for common sunscreen agents: application to in vivo assessment of skin penetration and systemic absorption in human volunteers. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 803, 225–231. - PubMed
-
- Li J., Ma L.Y., Xu L. (2016) Transformation of benzophenone-type UV filters by chlorine: kinetics, products identification and toxicity assessments. Journal of Hazardous Materials, 311, 263–272. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

