Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 28977399
- PMCID: PMC5861900
- DOI: 10.1093/gerona/glx137
Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
Background: Aging frailty, characterized by decreased physical and immunological functioning, is associated with stem cell depletion. Human allogeneic mesenchymal stem cells (allo-hMSCs) exert immunomodulatory effects and promote tissue repair.
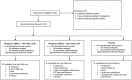
Methods: This is a randomized, double-blinded, dose-finding study of intravenous allo-hMSCs (100 or 200-million [M]) vs placebo delivered to patients (n = 30, mean age 75.5 ± 7.3) with frailty. The primary endpoint was incidence of treatment-emergent serious adverse events (TE-SAEs) at 1-month postinfusion. Secondary endpoints included physical performance, patient-reported outcomes, and immune markers of frailty measured at 6 months postinfusion.
Results: No therapy-related TE-SAEs occurred at 1 month. Physical performance improved preferentially in the 100M-group; immunologic improvement occurred in both the 100M- and 200M-groups. The 6-minute walk test, short physical performance exam, and forced expiratory volume in 1 second improved in the 100M-group (p = .01), not in the 200M- or placebo groups. The female sexual quality of life questionnaire improved in the 100M-group (p = .03). Serum TNF-α levels decreased in the 100M-group (p = .03). B cell intracellular TNF-α improved in both the 100M- (p < .0001) and 200M-groups (p = .002) as well as between groups compared to placebo (p = .003 and p = .039, respectively). Early and late activated T-cells were also reduced by MSC therapy.
Conclusion: Intravenous allo-hMSCs were safe in individuals with aging frailty. Treated groups had remarkable improvements in physical performance measures and inflammatory biomarkers, both of which characterize the frailty syndrome. Given the excellent safety and efficacy profiles demonstrated in this study, larger clinical trials are warranted to establish the efficacy of hMSCs in this multisystem disorder.
Clinical trial registration: www.clinicaltrials.gov: CRATUS (#NCT02065245).
Keywords: Immunomodulation; Regenerative medicine; Tumor necrosis factor-α.
© The Author 2017. Published by Oxford University Press on behalf of The Gerontological Society of America.
Figures



References
-
- Walston J, Hadley EC, Ferrucci L et al. . Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi:10.1111/j.1532-5415.2006.00745.x - PubMed
-
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi:10.1111/j.1532-5415.2010.02764.x - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

