A sequence-specific core promoter-binding transcription factor recruits TRF2 to coordinately transcribe ribosomal protein genes
- PMID: 28977400
- PMCID: PMC5737516
- DOI: 10.1093/nar/gkx676
A sequence-specific core promoter-binding transcription factor recruits TRF2 to coordinately transcribe ribosomal protein genes
Abstract
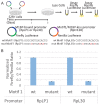
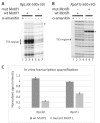
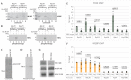
Ribosomal protein (RP) genes must be coordinately expressed for proper assembly of the ribosome yet the mechanisms that control expression of RP genes in metazoans are poorly understood. Recently, TATA-binding protein-related factor 2 (TRF2) rather than the TATA-binding protein (TBP) was found to function in transcription of RP genes in Drosophila. Unlike TBP, TRF2 lacks sequence-specific DNA binding activity, so the mechanism by which TRF2 is recruited to promoters is unclear. We show that the transcription factor M1BP, which associates with the core promoter region, activates transcription of RP genes. Moreover, M1BP directly interacts with TRF2 to recruit it to the RP gene promoter. High resolution ChIP-exo was used to analyze in vivo the association of M1BP, TRF2 and TFIID subunit, TAF1. Despite recent work suggesting that TFIID does not associate with RP genes in Drosophila, we find that TAF1 is present at RP gene promoters and that its interaction might also be directed by M1BP. Although M1BP associates with thousands of genes, its colocalization with TRF2 is largely restricted to RP genes, suggesting that this combination is key to coordinately regulating transcription of the majority of RP genes in Drosophila.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Hu H., Li X.. Transcriptional regulation in eukaryotic ribosomal protein genes. Genomics. 2007; 90:421–423. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

