Computational characterization of chromatin domain boundary-associated genomic elements
- PMID: 28977568
- PMCID: PMC5737353
- DOI: 10.1093/nar/gkx738
Computational characterization of chromatin domain boundary-associated genomic elements
Abstract
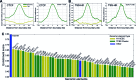
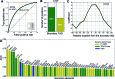
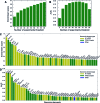
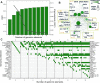
Topologically associated domains (TADs) are 3D genomic structures with high internal interactions that play important roles in genome compaction and gene regulation. Their genomic locations and their association with CCCTC-binding factor (CTCF)-binding sites and transcription start sites (TSSs) were recently reported. However, the relationship between TADs and other genomic elements has not been systematically evaluated. This was addressed in the present study, with a focus on the enrichment of these genomic elements and their ability to predict the TAD boundary region. We found that consensus CTCF-binding sites were strongly associated with TAD boundaries as well as with the transcription factors (TFs) Zinc finger protein (ZNF)143 and Yin Yang (YY)1. TAD boundary-associated genomic elements include DNase I-hypersensitive sites, H3K36 trimethylation, TSSs, RNA polymerase II, and TFs such as Specificity protein 1, ZNF274 and SIX homeobox 5. Computational modeling with these genomic elements suggests that they have distinct roles in TAD boundary formation. We propose a structural model of TAD boundaries based on these findings that provides a basis for studying the mechanism of chromatin structure formation and gene regulation.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Luger K., Mäder A.W., Richmond R.K., Sargent D.F., Richmond T.J.. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997; 389:251–260. - PubMed
-
- Cremer T., Cremer C.. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2001; 2:292–301. - PubMed
-
- Maeshima K., Ide S., Hibino K., Sasai M.. Liquid-like behavior of chromatin. Curr. Opin. Genet. Dev. 2016; 37:36–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

