Dipeptidyl Peptidase-4 Inhibition With Saxagliptin Ameliorates Angiotensin II-Induced Cardiac Diastolic Dysfunction in Male Mice
- PMID: 28977602
- PMCID: PMC5659692
- DOI: 10.1210/en.2017-00416
Dipeptidyl Peptidase-4 Inhibition With Saxagliptin Ameliorates Angiotensin II-Induced Cardiac Diastolic Dysfunction in Male Mice
Abstract
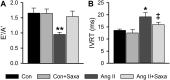
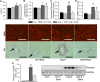
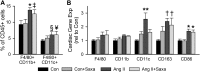
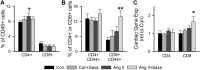
Activation of the renin-angiotensin-aldosterone system is common in hypertension and obesity and contributes to cardiac diastolic dysfunction, a condition for which no treatment currently exists. In light of recent reports that antihyperglycemia incretin enhancing dipeptidyl peptidase (DPP)-4 inhibitors exert cardioprotective effects, we examined the hypothesis that DPP-4 inhibition with saxagliptin (Saxa) attenuates angiotensin II (Ang II)-induced cardiac diastolic dysfunction. Male C57BL/6J mice were infused with either Ang II (500 ng/kg/min) or vehicle for 3 weeks receiving either Saxa (10 mg/kg/d) or placebo during the final 2 weeks. Echocardiography revealed Ang II-induced diastolic dysfunction, evidenced by impaired septal wall motion and prolonged isovolumic relaxation, coincident with aortic stiffening. Ang II induced cardiac hypertrophy, coronary periarterial fibrosis, TRAF3-interacting protein 2 (TRAF3IP2)-dependent proinflammatory signaling [p-p65, p-c-Jun, interleukin (IL)-17, IL-18] associated with increased cardiac macrophage, but not T cell, gene expression. Flow cytometry revealed Ang II-induced increases of cardiac CD45+F4/80+CD11b+ and CD45+F4/80+CD11c+ macrophages and CD45+CD4+ lymphocytes. Treatment with Saxa reduced plasma DPP-4 activity and abrogated Ang II-induced cardiac diastolic dysfunction independent of aortic stiffening or blood pressure. Furthermore, Saxa attenuated Ang II-induced periarterial fibrosis and cardiac inflammation, but not hypertrophy or cardiac macrophage infiltration. Analysis of Saxa-induced changes in cardiac leukocytes revealed Saxa-dependent reduction of the Ang II-mediated increase of cardiac CD11c messenger RNA and increased cardiac CD8 gene expression and memory CD45+CD8+CD44+ lymphocytes. In summary, these results demonstrate that DPP-4 inhibition with Saxa prevents Ang II-induced cardiac diastolic dysfunction, fibrosis, and inflammation associated with unique shifts in CD11c-expressing leukocytes and CD8+ lymphocytes.
Copyright © 2017 Endocrine Society.
Figures


Similar articles
-
DPP4 inhibition mitigates ANG II-mediated kidney immune activation and injury in male mice.Am J Physiol Renal Physiol. 2021 Mar 1;320(3):F505-F517. doi: 10.1152/ajprenal.00565.2020. Epub 2021 Feb 1. Am J Physiol Renal Physiol. 2021. PMID: 33522410 Free PMC article.
-
SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor.Cardiovasc Drugs Ther. 2017 Apr;31(2):119-132. doi: 10.1007/s10557-017-6725-2. Cardiovasc Drugs Ther. 2017. PMID: 28447181
-
Cardiac DPP-4 inhibition by saxagliptin ameliorates isoproterenol-induced myocardial remodeling and cardiac diastolic dysfunction in rats.J Pharmacol Sci. 2016 Sep;132(1):65-70. doi: 10.1016/j.jphs.2016.08.008. Epub 2016 Sep 8. J Pharmacol Sci. 2016. PMID: 27666017
-
Clinical Pharmacokinetics and Pharmacodynamics of Saxagliptin, a Dipeptidyl Peptidase-4 Inhibitor.Clin Pharmacokinet. 2017 Jan;56(1):11-24. doi: 10.1007/s40262-016-0421-4. Clin Pharmacokinet. 2017. PMID: 27282159 Review.
-
Pathophysiology of Angiotensin II-Mediated Hypertension, Cardiac Hypertrophy, and Failure: A Perspective from Macrophages.Cells. 2024 Dec 4;13(23):2001. doi: 10.3390/cells13232001. Cells. 2024. PMID: 39682749 Free PMC article. Review.
Cited by
-
Roles and Mechanisms of Dipeptidyl Peptidase 4 Inhibitors in Vascular Aging.Front Endocrinol (Lausanne). 2021 Aug 17;12:731273. doi: 10.3389/fendo.2021.731273. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34489872 Free PMC article. Review.
-
Long-Term Dipeptidyl Peptidase 4 Inhibition Worsens Hypertension and Renal and Cardiac Abnormalities in Obese Spontaneously Hypertensive Heart Failure Rats.J Am Heart Assoc. 2021 Mar 16;10(6):e020088. doi: 10.1161/JAHA.120.020088. Epub 2021 Mar 8. J Am Heart Assoc. 2021. PMID: 33682436 Free PMC article.
-
Integrated miRNA-mRNA networks underlie attenuation of chronic β-adrenergic stimulation-induced cardiac remodeling by minocycline.Physiol Genomics. 2024 Apr 1;56(4):360-366. doi: 10.1152/physiolgenomics.00140.2023. Epub 2024 Feb 5. Physiol Genomics. 2024. PMID: 38314697 Free PMC article.
-
Multi-omic analysis of the cardiac cellulome defines a vascular contribution to cardiac diastolic dysfunction in obese female mice.Basic Res Cardiol. 2023 Mar 29;118(1):11. doi: 10.1007/s00395-023-00983-6. Basic Res Cardiol. 2023. PMID: 36988733 Free PMC article.
-
Mineralocorticoid Receptor in Myeloid Cells Mediates Angiotensin II-Induced Vascular Dysfunction in Female Mice.Front Physiol. 2021 Mar 29;12:588358. doi: 10.3389/fphys.2021.588358. eCollection 2021. Front Physiol. 2021. PMID: 33854438 Free PMC article.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. - PubMed
-
- Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, Jaber WA. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125(6):782–788. - PubMed
-
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

