Andrographolide inhibits arrhythmias and is cardioprotective in rabbits
- PMID: 28977859
- PMCID: PMC5617419
- DOI: 10.18632/oncotarget.18051
Andrographolide inhibits arrhythmias and is cardioprotective in rabbits
Abstract
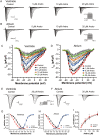
Andrographolide has a protective effect on the cardiovascular system. To study its cardic-electrophysiological effects, action potentials and voltage-gated Na+ (INa), Ca2+ (ICaL), and K+ (IK1, IKr, Ito and IKur) currents were recorded using whole-cell patch clamp and current clamp techniques. Additionally, the effects of andrographolide on aconitine-induced arrhythmias were assessed on electrocardiograms in vivo. We found that andrographolide shortened action potential duration and reduced maximum upstroke velocity in rabbit left ventricular and left atrial myocytes. Andrographolide attenuated rate-dependence of action potential duration, and reduced or abolished delayed afterdepolarizations and triggered activities induced by isoproterenol (1 μM) and high calcium ([Ca2+]o=3.6 mM) in left ventricular myocytes. Andrographolide also concentration-dependently inhibited INa and ICaL, but had no effect on Ito, IKur, IK1, or IKr in rabbit left ventricular and left atrial myocytes. Andrographolide treatment increased the time and dosage thresholds of aconitine-induced arrhythmias, and reduced arrhythmia incidence and mortality in rabbits. Our results indicate that andrographolide inhibits cellular arrhythmias (delayed afterdepolarizations and triggered activities) and aconitine-induced arrhythmias in vivo, and these effects result from INa and ICaL inhibition. Andrographolide may be useful as a class I and IV antiarrhythmic therapeutic.
Keywords: L-type calcium current; action potential; andrographolide; arrhythmia; sodium current.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures





Similar articles
-
Icariin, a Novel Blocker of Sodium and Calcium Channels, Eliminates Early and Delayed Afterdepolarizations, As Well As Triggered Activity, in Rabbit Cardiomyocytes.Front Physiol. 2017 May 29;8:342. doi: 10.3389/fphys.2017.00342. eCollection 2017. Front Physiol. 2017. PMID: 28611679 Free PMC article.
-
Antiarrhythmic effect of crotonoside by regulating sodium and calcium channels in rabbit ventricular myocytes.Life Sci. 2020 Mar 1;244:117333. doi: 10.1016/j.lfs.2020.117333. Epub 2020 Jan 18. Life Sci. 2020. PMID: 31962132
-
Barbaloin inhibits ventricular arrhythmias in rabbits by modulating voltage-gated ion channels.Acta Pharmacol Sin. 2018 Mar;39(3):357-370. doi: 10.1038/aps.2017.93. Epub 2017 Oct 26. Acta Pharmacol Sin. 2018. PMID: 29072259 Free PMC article.
-
[Effects of BmKIM on sodium current of isolated cardiomyocytes, transmembrane action potential and aconitine induced arrhythmia in vivo in rabbits].Zhonghua Xin Xue Guan Bing Za Zhi. 2009 Feb;37(2):102-7. Zhonghua Xin Xue Guan Bing Za Zhi. 2009. PMID: 19719982 Chinese.
-
Therapeutic Effects of Wenxin Keli in Cardiovascular Diseases: An Experimental and Mechanism Overview.Front Pharmacol. 2018 Sep 5;9:1005. doi: 10.3389/fphar.2018.01005. eCollection 2018. Front Pharmacol. 2018. PMID: 30233380 Free PMC article. Review.
Cited by
-
Mechanism-based targeting of cardiac arrhythmias by phytochemicals and medicinal herbs: A comprehensive review of preclinical and clinical evidence.Front Cardiovasc Med. 2022 Sep 29;9:990063. doi: 10.3389/fcvm.2022.990063. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36247473 Free PMC article. Review.
-
A Bitter Taste in Your Heart.Front Physiol. 2020 May 8;11:431. doi: 10.3389/fphys.2020.00431. eCollection 2020. Front Physiol. 2020. PMID: 32457649 Free PMC article. Review.
-
Andrographolide protects against atrial fibrillation by alleviating oxidative stress injury and promoting impaired mitochondrial bioenergetics.J Zhejiang Univ Sci B. 2023 Jul 15;24(7):632-649. doi: 10.1631/jzus.B2300086. J Zhejiang Univ Sci B. 2023. PMID: 37455139 Free PMC article.
-
Andrographolide Protects against Aortic Banding-Induced Experimental Cardiac Hypertrophy by Inhibiting MAPKs Signaling.Front Pharmacol. 2017 Nov 14;8:808. doi: 10.3389/fphar.2017.00808. eCollection 2017. Front Pharmacol. 2017. PMID: 29184496 Free PMC article.
References
-
- Kishore V, Yarla NS, Bishayee A, Putta S, Malla R, Neelapu NR, Challa S, Das S, Shiralgi Y, Hegde G, Dhananjaya BL. Multi-targeting andrographolide and its natural analogs as potential therapeutic agents. Curr Top Med Chem. 2017;17:845–857. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

