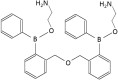
The 2-aminoethoxydiphenyl borate analog, DPB161 blocks store-operated Ca2+ entry in acutely dissociated rat submandibular cells
- PMID: 28977884
- PMCID: PMC5617444
- DOI: 10.18632/oncotarget.18623
The 2-aminoethoxydiphenyl borate analog, DPB161 blocks store-operated Ca2+ entry in acutely dissociated rat submandibular cells
Abstract
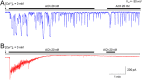
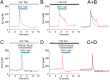
Cellular Ca2+ signals play a critical role in cell physiology and pathology. In most non-excitable cells, store-operated Ca2+ entry (SOCE) is an important mechanism by which intracellular Ca2+ signaling is regulated. However, few drugs can selectively modulate SOCE. 2-Aminoethoxydiphenyl borate (2APB) and its analogs (DPB162 and DPB163) have been reported to inhibit SOCE. Here, we examined the effects of another 2-APB analog, DPB161 on SOCE in acutely-isolated rat submandibular cells. Both patch-clamp recordings and Ca2+ imaging showed that upon removal of extracellular Ca2+ ([Ca2+]o=0), rat submandibular cells were unable to maintain ACh-induced Ca2+ oscillations, but restoration of [Ca2+]o to refill Ca2+ stores enable recovery of these Ca2+ oscillations. However, addition of 50 μM DPB161 with [Ca2+]o to extracellular solution prevented the refilling of Ca2+ store. Fura-2 Ca2+ imaging showed that DPB161 inhibited SOCE in a concentration-dependent manner. After depleting Ca2+ stores by thapsigargin treatment, bath perfusion of 1 mM Ca2+ induced [Ca2+]i elevation in a manner that was prevented by DPB161. Collectively, these results show that the 2-APB analog DPB161 blocks SOCE in rat submandibular cells, suggesting that this compound can be developed as a pharmacological tool for the study of SOCE function and as a new therapeutic agent for treating SOCE-associated disorders.
Keywords: 2-aminoethoxydiphenyl borate; Ca2+ oscillations; DPB161; rat submandibular cells; store-operated Ca2+ entry (SOCE).
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures



Similar articles
-
DPB162-AE, an inhibitor of store-operated Ca2+ entry, can deplete the endoplasmic reticulum Ca2+ store.Cell Calcium. 2017 Mar;62:60-70. doi: 10.1016/j.ceca.2017.01.015. Epub 2017 Feb 1. Cell Calcium. 2017. PMID: 28196740
-
Ca2+ signaling mediated by IP3-dependent Ca2+ releasing and store-operated Ca2+ channels in rat odontoblasts.J Bone Miner Res. 2003 Jan;18(1):30-8. doi: 10.1359/jbmr.2003.18.1.30. J Bone Miner Res. 2003. PMID: 12510803
-
Pharmacological profile of store-operated Ca(2+) entry in intrapulmonary artery smooth muscle cells.Eur J Pharmacol. 2008 Apr 14;584(1):10-20. doi: 10.1016/j.ejphar.2008.01.018. Epub 2008 Jan 26. Eur J Pharmacol. 2008. PMID: 18308301
-
Store-operated calcium entry mediates intracellular alkalinization, ERK1/2, and Akt/PKB phosphorylation in bovine neutrophils.J Leukoc Biol. 2007 Nov;82(5):1266-77. doi: 10.1189/jlb.0307196. Epub 2007 Aug 7. J Leukoc Biol. 2007. PMID: 17684040
-
Historical Overview of Store-Operated Ca(2+) Entry.Adv Exp Med Biol. 2016;898:3-24. doi: 10.1007/978-3-319-26974-0_1. Adv Exp Med Biol. 2016. PMID: 27161222 Review.
References
-
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993;14:746–57. - PubMed
-
- Petersen OH, Petersen CC, Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. - PubMed
-
- Petersen OH, Tepikin A, Park MK. The endoplasmic reticulum: one continuous or several separate Ca(2+) stores? Trends Neurosci. 2001;24:271–6. - PubMed
-
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

