Overexpression of stathmin plays a pivotal role in the metastasis of esophageal squamous cell carcinoma
- PMID: 28977901
- PMCID: PMC5617461
- DOI: 10.18632/oncotarget.18687
Overexpression of stathmin plays a pivotal role in the metastasis of esophageal squamous cell carcinoma
Abstract
Purpose: Esophageal squamous cell carcinoma (ESCC) is a serious malignant tumor that affects human health. We analyzed the correlation between serum stathmin level and ESCC and elucidated the molecular mechanisms of stathmin's promotion of ESCC cell invasion and metastasis.
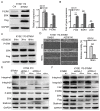
Methods: Stathmin level in ESCC and healthy control serum were detected by enzyme-linked immunosorbent assay (ELISA), and the clinical parameters were analyzed. We established ESCC cells with stathmin overexpression or knockdown and then evaluated the effects of stathmin on invasion and metastasis in ESCC. Differentially expressed genes were analyzed by Human Transcriptome Array and confirmed by RT-PCR. The expression levels of the integrin family, focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK) were detected by immunoblotting.
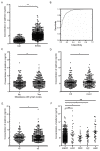
Results: Serum levels of stathmin were significantly higher in ESCC than in control serum and associated with lymph node metastasis, tumor stage and size. Furthermore, we found that stathmin promoted migration and invasion of ESCC cells in vitro and in vivo. In addition, we confirmed that the activation of the integrinα5β1/FAK/ERK pathway is increased in stathmin-overexpression cells and accelerates cell motility by enhancing cell adhesion ability.
Conclusion: Stathmin may predict a potential metastasis biomarker for ESCC.
Keywords: ERK; ESCC; FAK; integrinα5β1; stathmin.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have declared that no competing interests exist.
Figures







References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Qiu H, Mao Y, Gu Y, Zhu J, Wang Y, Zeng J, Huang N, Liu Q, Yang Y. The potential of photodynamic therapy to treat esophageal candidiasis coexisting with esophageal cancer. J Photochem Photobiol B. 2014;130:305–309. - PubMed
-
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

