MOC31PE immunotoxin - targeting peritoneal metastasis from epithelial ovarian cancer
- PMID: 28977905
- PMCID: PMC5617465
- DOI: 10.18632/oncotarget.18694
MOC31PE immunotoxin - targeting peritoneal metastasis from epithelial ovarian cancer
Abstract
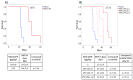
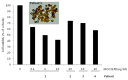
Peritoneal metastasis (PM) is an important feature of epithelial ovarian cancer (EOC) and is a frequent site of drug resistant disease recurrence, identifying PM-EOC an important clinical challenge. The MOC31PE immunotoxin targets and kills tumor cells expressing the epithelial cell adhesion molecule (EpCAM), which is highly expressed in EOC, and MOC31PE is being investigated for use in treatment of PM-EOC. The efficacy of MOC31PE treatment alone and in combination with cytotoxic drugs was investigated in two human EpCAM expressing EOC cell lines, B76 and MDHA-2774, in vitro and in corresponding mouse models mimicking PM-EOC. MOC31PE efficaciously killed tumor cells alone and showed equal or superior activity in vitro (paclitaxel, cisplatin, carboplatin) and in vivo (paclitaxel, mitomycin C) compared to the investigated cytotoxic drugs. Additive, or importantly, no antagonistic effects were observed in combination experiments. In ex vivo cell culture, the cytotoxic effect of MOC31PE was studied on freshly isolated surgical EOC samples. All investigated fresh EOC samples expressed EpCAM and MOC31PE effectively reduced cell viability in ex vivo cultures. In conclusion, these results, together with our previous preclinical and clinical experience, support development of MOC31PE for treatment of PM-EOC in combination with currently used cytotoxic drugs.
Keywords: EpCAM; MOC31PE immunotoxin; animal models; chemotherapy; peritoneal metastasis of epithelial ovarian cancer.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed.
Figures



Similar articles
-
Immunotoxin targeting EpCAM effectively inhibits peritoneal tumor growth in experimental models of mucinous peritoneal surface malignancies.Int J Cancer. 2013 Sep 15;133(6):1497-506. doi: 10.1002/ijc.28158. Epub 2013 Apr 17. Int J Cancer. 2013. PMID: 23494569
-
Novel Treatment with Intraperitoneal MOC31PE Immunotoxin in Colorectal Peritoneal Metastasis: Results From the ImmunoPeCa Phase 1 Trial.Ann Surg Oncol. 2017 Jul;24(7):1916-1922. doi: 10.1245/s10434-017-5814-6. Epub 2017 Feb 21. Ann Surg Oncol. 2017. PMID: 28224367 Clinical Trial.
-
The MOC31PE immunotoxin reduces cell migration and induces gene expression and cell death in ovarian cancer cells.J Ovarian Res. 2014 Feb 15;7:23. doi: 10.1186/1757-2215-7-23. J Ovarian Res. 2014. PMID: 24528603 Free PMC article.
-
Phase I trial of EpCAM-targeting immunotoxin MOC31PE, alone and in combination with cyclosporin.Br J Cancer. 2015 Dec 1;113(11):1548-55. doi: 10.1038/bjc.2015.380. Epub 2015 Nov 10. Br J Cancer. 2015. PMID: 26554649 Free PMC article. Clinical Trial.
-
Epithelial cell-adhesion molecule-directed trifunctional antibody immunotherapy for symptom management of advanced ovarian cancer.Clin Pharmacol. 2013 Oct 3;5(Suppl 1):55-61. doi: 10.2147/CPAA.S45885. Clin Pharmacol. 2013. PMID: 24124397 Free PMC article. Review.
Cited by
-
Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years?Cancer Metastasis Rev. 2020 Sep;39(3):969-987. doi: 10.1007/s10555-020-09898-3. Cancer Metastasis Rev. 2020. PMID: 32507912 Free PMC article. Review.
-
Immunotherapy for Peritoneal Carcinomatosis: Challenges and Prospective Outcomes.Cancers (Basel). 2023 Apr 20;15(8):2383. doi: 10.3390/cancers15082383. Cancers (Basel). 2023. PMID: 37190310 Free PMC article. Review.
-
Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1.Int J Mol Sci. 2020 May 7;21(9):3310. doi: 10.3390/ijms21093310. Int J Mol Sci. 2020. PMID: 32392820 Free PMC article.
-
Anti-Angiogenic Treatment in Pseudomyxoma Peritonei-Still a Strong Preclinical Rationale.Cancers (Basel). 2021 Jun 5;13(11):2819. doi: 10.3390/cancers13112819. Cancers (Basel). 2021. PMID: 34198773 Free PMC article.
References
-
- Andersson Y, Juell S, Fodstad O. Downregulation of the antiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor-Pseudomonas exotoxin a immunotoxin. Int J Cancer. 2004;112:475–483. - PubMed
-
- Andersson Y, Le H, Juell S, Fodstad O. AMP-activated protein kinase protects against anti-epidermal growth factor receptor-Pseudomonas exotoxin A immunotoxin-induced MA11 breast cancer cell death. Mol Cancer Ther. 2006;5:1050–1059. - PubMed
-
- Aoki Y, Kurata H, Watanabe M, Fujita K, Tanaka K. Combination chemotherapy with irinotecan hydrochloride (CPT-11) and mitomycin C in platinum-refractory ovarian cancer. Am J Clin Oncol. 2004;27:461–464. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

