Trypsin-protease activated receptor-2 signaling contributes to pancreatic cancer pain
- PMID: 28977906
- PMCID: PMC5617466
- DOI: 10.18632/oncotarget.18696
Trypsin-protease activated receptor-2 signaling contributes to pancreatic cancer pain
Abstract
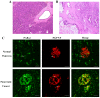
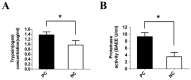
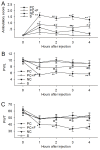
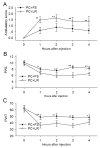
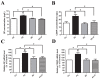
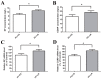
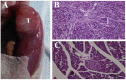
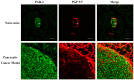
Pain treatment is a critical aspect of pancreatic cancer patient clinical care. This study investigated the role of trypsin-protease activated receptor-2 (PAR-2) in pancreatic cancer pain. Pancreatic tissue samples were collected from pancreatic cancer (n=22) and control patients (n=22). Immunofluorescence analyses confirmed colocalization of PAR-2 and neuronal markers in pancreatic cancer tissues. Trypsin levels and protease activities were higher in pancreatic cancer tissue specimens than in the controls. Supernatants from cultured human pancreatic cancer tissues (PC supernatants) induced substance P and calcitonin gene-related peptide release in dorsal root ganglia (DRG) neurons, and FS-NH2, a selective PAR-2 antagonist, inhibited this effect. A BALB/c nude mouse orthotopic tumor model was used to confirm the role of PAR-2 signaling in pancreatic cancer visceral pain, and male Sprague-Dawley rats were used to assess ambulatory pain. FS-NH2 treatment decreased hunch scores, mechanical hyperalgesia, and visceromotor reflex responses in tumor-bearing mice. In rats, subcutaneous injection of PC supernatant induced pain behavior, which was alleviated by treatment with FS-NH2 or FUT-175, a broad-spectrum serine protease inhibitor. Our findings suggest that trypsin-PAR-2 signaling contributes to pancreatic cancer pain in vivo. Treatment strategies targeting PAR-2 or its downstream signaling molecules might effectively relieve pancreatic cancer pain.
Keywords: pain; pancreatic cancer; pancreatic cancer pain model; protease activated receptor-2; trypsin.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures
Similar articles
-
The C-terminus of murine S100A9 protein inhibits hyperalgesia induced by the agonist peptide of protease-activated receptor 2 (PAR2).Br J Pharmacol. 2006 Oct;149(4):374-84. doi: 10.1038/sj.bjp.0706884. Epub 2006 Sep 11. Br J Pharmacol. 2006. PMID: 16967049 Free PMC article.
-
Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia.Am J Physiol Gastrointest Liver Physiol. 2008 May;294(5):G1288-98. doi: 10.1152/ajpgi.00002.2008. Epub 2008 Mar 6. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18325985
-
Potentiation of the P2X3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain.Eur J Neurosci. 2012 Aug;36(3):2293-301. doi: 10.1111/j.1460-9568.2012.08142.x. Epub 2012 May 23. Eur J Neurosci. 2012. PMID: 22616675
-
Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity.Neurogastroenterol Motil. 2009 Nov;21(11):1189-e107. doi: 10.1111/j.1365-2982.2009.01310.x. Epub 2009 Apr 20. Neurogastroenterol Motil. 2009. PMID: 19413681
-
Is There a Trojan Horse to Aggressive Pancreatic Cancer Biology? A Review of the Trypsin-PAR2 Axis to Proliferation, Early Invasion, and Metastasis.J Pancreat Cancer. 2020 Feb 6;6(1):12-20. doi: 10.1089/pancan.2019.0014. eCollection 2020. J Pancreat Cancer. 2020. PMID: 32064449 Free PMC article. Review.
Cited by
-
Resolving the conflicts around Par2 opposing roles in regeneration by comparing immune-mediated and toxic-induced injuries.Inflamm Regen. 2022 Nov 29;42(1):52. doi: 10.1186/s41232-022-00238-2. Inflamm Regen. 2022. PMID: 36447218 Free PMC article.
-
New therapies to relieve pain: The search for more efficient and safer alternatives to opioid pain killers.EMBO Rep. 2018 Oct;19(10):e46925. doi: 10.15252/embr.201846925. Epub 2018 Sep 17. EMBO Rep. 2018. PMID: 30224409 Free PMC article.
-
PAR-2 promotes cell proliferation, migration, and invasion through activating PI3K/AKT signaling pathway in oral squamous cell carcinoma.Biosci Rep. 2019 Jul 5;39(7):BSR20182476. doi: 10.1042/BSR20182476. Print 2019 Jul 31. Biosci Rep. 2019. Retraction in: Biosci Rep. 2024 Jul 31;44(7):BSR-2018-2476_RET. doi: 10.1042/BSR-2018-2476_RET. PMID: 31213575 Free PMC article. Retracted.
-
PAR2: The Cornerstone of Pancreatic Diseases.Physiol Res. 2022 Nov 28;71(5):583-596. doi: 10.33549/physiolres.934931. Epub 2022 Sep 8. Physiol Res. 2022. PMID: 36073735 Free PMC article. Review.
-
Reactive oxygen species and enzyme dual-responsive biocompatible drug delivery system for targeted tumor therapy.J Control Release. 2020 Aug 10;324:330-340. doi: 10.1016/j.jconrel.2020.05.031. Epub 2020 May 23. J Control Release. 2020. PMID: 32450093 Free PMC article.
References
-
- Mekaroonkamol P, Willingham FF, Chawla S. Endoscopic management of pain in pancreatic cancer. JOP. 2015;16:33–40. - PubMed
-
- Sakamoto H, Kitano M, Komaki T, Imai H, Kamata K, Kudo M. Endoscopic ultrasound-guided neurolysis in pancreatic cancer. Pancreatology. 2011;11:52–58. - PubMed
-
- Oliveira SM, Silva CR, Ferreira J. Critical role of protease-activated receptor 2 activation by mast cell tryptase in the development of postoperative pain. Anesthesiology. 2013;118:679–690. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

