2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenyl carbamoylsulfanyl] propionic acid, a glutathione reductase inhibitor, induces G2/M cell cycle arrest through generation of thiol oxidative stress in human esophageal cancer cells
- PMID: 28977909
- PMCID: PMC5617469
- DOI: 10.18632/oncotarget.18705
2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenyl carbamoylsulfanyl] propionic acid, a glutathione reductase inhibitor, induces G2/M cell cycle arrest through generation of thiol oxidative stress in human esophageal cancer cells
Abstract
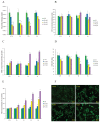
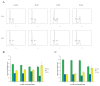
Esophageal squamous cell carcinoma (ESCC) is a highly malignant cancer with poor response to both of chemotherapy and radiotherapy. 2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenyl carbamoylsulfanyl] propionic acid (2-AAPA), an irreversible inhibitor of glutathione reductase (GR), is able to induce intracellular oxidative stress, and has shown anticancer activity in many cancer cell lines. In this study, we investigated the effects of 2-AAPA on the cell proliferation, cell cycle and apoptosis and aimed to explore its mechanism of action in human esophageal cancer TE-13 cells. It was found that 2-AAPA inhibited growth of ESCC cells in a dose-dependent manner and it did not deplete reduced glutathione (GSH), but significantly increased the oxidized form glutathione (GSSG), resulting in decreased GSH/GSSG ratio. In consequence, significant reactive oxygen species (ROS) production was observed. The flow cytometric analysis revealed that 2-AAPA inhibited growth of esophageal cancer cells through arresting cell cycle in G2/M phase, but apoptosis-independent mechanism. The G2/M arrest was partially contributed by down-regulation of protein expression of Cdc-25c and up-regulation of phosphorylated Cdc-2 (Tyr15), Cyclin B1 (Ser147) and p53. Meanwhile, 2-AAPA-induced thiol oxidative stress led to increased protein S-glutathionylation, which resulted in α-tubulin S-glutathionylation-dependent depolymerization of microtubule in the TE-13 cells. In conclusion, we identified that 2-AAPA as an effective thiol oxidative stress inducer and proliferation of TE-13 cells were suppressed by G2/M phase cell cycle arrest, mainly, through α-tubulin S-glutathionylation-mediated microtubule depolymerization. Our results may introduce new target and approach for esophageal cancer therapy through generation of GR-mediated thiol oxidative stress.
Keywords: S-glutathionylation; cell cycle arrest; glutathione reductase; microtubule depolymerization; oxidative stress.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed.
Figures





References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Ren QG, Yang SL, Hu JL, Li PD, Chen YS, Wang QS. Evaluation of HO-1 expression, cellular ROS production, cellular proliferation and cellular apoptosis in human esophageal squamous cell carcinoma tumors and cell lines. Oncol Rep. 2016;35:2270–2276. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

