Tumor p38MAPK signaling enhances breast carcinoma vascularization and growth by promoting expression and deposition of pro-tumorigenic factors
- PMID: 28977919
- PMCID: PMC5617479
- DOI: 10.18632/oncotarget.18755
Tumor p38MAPK signaling enhances breast carcinoma vascularization and growth by promoting expression and deposition of pro-tumorigenic factors
Abstract
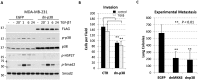
The breast carcinoma microenvironment strikingly influences cancer progression and response to therapy. Various cell types in the carcinoma microenvironment show significant activity of p38 mitogen-activated protein kinase (MAPK), although the role of p38MAPK in breast cancer progression is still poorly understood. The present study examined the contribution of tumor p38MAPK to breast carcinoma microenvironment and metastatic capacity. Inactivation of p38MAPK signaling in metastatic breast carcinoma cells was achieved by forced expression of the kinase-inactive mutant of p38/MAPK14 (a dominant-negative p38, dn-p38). Disruption of tumor p38MAPK signaling reduced growth and metastases of breast carcinoma xenografts. Importantly, dn-p38 markedly decreased tumor blood-vessel density and lumen sizes. Mechanistic studies revealed that p38 controls expression of pro-angiogenic extracellular factors such as matrix protein Fibronectin and cytokines VEGFA, IL8, and HBEGF. Tumor-associated fibroblasts enhanced tumor growth and vasculature as well as increased expression of the pro-angiogenic factors. These effects were blunted by dn-p38. Metadata analysis showed elevated expression of p38 target genes in breast cancers and this was an unfavorable marker of disease recurrence and poor-outcome. Thus, our study demonstrates that tumor p38MAPK signaling promotes breast carcinoma growth, invasive and metastatic capacities. Importantly, p38 enhances carcinoma vascularization by facilitating expression and deposition of pro-angiogenic factors. These results argue that p38MAPK is a valuable target for anticancer therapy affecting tumor vasculature. Anti-p38 drugs may provide new therapeutic strategies against breast cancer, including metastatic disease.
Keywords: angiogenesis; breast cancer; fibronectin; p38MAPK; tumor microenvironment.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures





Similar articles
-
TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association.BMC Cancer. 2018 Jun 19;18(1):670. doi: 10.1186/s12885-018-4587-z. BMC Cancer. 2018. PMID: 29921235 Free PMC article.
-
[Correlation of p38 mitogen-activated protein kinase signal transduction pathway to uPA expression in breast cancer].Ai Zheng. 2007 Jan;26(1):48-53. Ai Zheng. 2007. PMID: 17222367 Chinese.
-
TGF-β autocrine pathway and MAPK signaling promote cell invasiveness and in vivo mammary adenocarcinoma tumor progression.Oncol Rep. 2012 Aug;28(2):567-75. doi: 10.3892/or.2012.1813. Epub 2012 May 14. Oncol Rep. 2012. PMID: 22614218 Free PMC article.
-
Functional Roles of JNK and p38 MAPK Signaling in Nasopharyngeal Carcinoma.Int J Mol Sci. 2022 Jan 20;23(3):1108. doi: 10.3390/ijms23031108. Int J Mol Sci. 2022. PMID: 35163030 Free PMC article. Review.
-
Current Insights of Inhibitors of p38 Mitogen-Activated Protein Kinase in Inflammation.Med Chem. 2021;17(6):555-575. doi: 10.2174/1573406416666200227122849. Med Chem. 2021. PMID: 32106802 Review.
Cited by
-
The endocrine disruptor cadmium modulates the androgen-estrogen receptors ratio and induces inflammatory cytokines in luminal (A) cell models of breast cancer.Endocrine. 2024 Mar;83(3):798-809. doi: 10.1007/s12020-023-03594-2. Epub 2023 Nov 18. Endocrine. 2024. PMID: 37979099 Free PMC article.
-
Novel Pathways for Targeting Tumor Angiogenesis in Metastatic Breast Cancer.Front Oncol. 2021 Dec 3;11:772305. doi: 10.3389/fonc.2021.772305. eCollection 2021. Front Oncol. 2021. PMID: 34926282 Free PMC article. Review.
-
Effects of Platycodon grandiflorus on doxorubicin resistance and epithelial-mesenchymal transition of breast cancer cells via the p38 mitogen-activated protein kinase pathway.J Mol Histol. 2024 Dec;55(6):1307-1314. doi: 10.1007/s10735-024-10271-9. Epub 2024 Sep 24. J Mol Histol. 2024. PMID: 39316256
-
Blockade of p38 kinase impedes the mobilization of protumorigenic myeloid populations to impact breast cancer metastasis.Int J Cancer. 2020 Oct 15;147(8):2279-2292. doi: 10.1002/ijc.33050. Epub 2020 May 28. Int J Cancer. 2020. PMID: 32452014 Free PMC article.
-
The p38 Pathway: From Biology to Cancer Therapy.Int J Mol Sci. 2020 Mar 11;21(6):1913. doi: 10.3390/ijms21061913. Int J Mol Sci. 2020. PMID: 32168915 Free PMC article. Review.
References
-
- Cancer Facts and Figures 2016. American Cancer Society; Atlanta, GA: 2016.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

