Chiral platinum (II)-4-(2,3-dihydroxypropyl)- formamide oxo-aporphine (FOA) complexes promote tumor cells apoptosis by directly targeting G-quadruplex DNA in vitro and in vivo
- PMID: 28977920
- PMCID: PMC5617480
- DOI: 10.18632/oncotarget.18778
Chiral platinum (II)-4-(2,3-dihydroxypropyl)- formamide oxo-aporphine (FOA) complexes promote tumor cells apoptosis by directly targeting G-quadruplex DNA in vitro and in vivo
Abstract
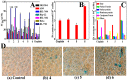
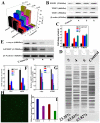
Three platinum(II) complexes, 4 (LC-004), 5 (LC-005), and 6 (LC-006), with the chiral FOA ligands R/S-(±)-FOA (1), R-(+)-FOA (2) and S-(-)-FOA (3), respectively, were synthesized and characterized. As potential anti-tumor agents, these complexes show higher cytotoxicity to BEL-7404 cells than the HL-7702 normal cells. They are potential telomerase inhibitors that target c-myc and human telomeric G-quadruplex DNA. Compared to complexes 4 and 5, 6 exhibited higher binding affinities towards telomeric, c-myc G-quadruplex DNA and caspase-3/9, thereby inducing senescence and apoptosis to a greater extent in tumor cells. Moreover, our in vivo studies showed that complex 6 can effectively inhibit tumor growth in the BEL-7404 and BEL-7402 xenograft mouse models and is less toxic than 5-fluorouracil and cisplatin. The effective inhibition of tumor growth is attributed to its interactions with 53BP1, TRF1, c-myc, TRF2, and hTERT. Thus, complex 6 can serve as a novel lead compound and a potential drug candidate for anticancer chemotherapy.
Keywords: G-quadruplex DNA; antitumor activity; chiral platinum(II) complex; oxoaporphine; telomerase.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declared that they have no conflicts of interest to this work.
Figures





Similar articles
-
Water-Soluble Ruthenium(II) Complexes with Chiral 4-(2,3-Dihydroxypropyl)-formamide Oxoaporphine (FOA): In Vitro and in Vivo Anticancer Activity by Stabilization of G-Quadruplex DNA, Inhibition of Telomerase Activity, and Induction of Tumor Cell Apoptosis.J Med Chem. 2015 Jun 11;58(11):4771-89. doi: 10.1021/acs.jmedchem.5b00444. Epub 2015 Jun 2. J Med Chem. 2015. PMID: 25988535
-
A platinum(II) complex of liriodenine from traditional Chinese medicine (TCM): Cell cycle arrest, cell apoptosis induction and telomerase inhibition activity via G-quadruplex DNA stabilization.J Inorg Biochem. 2014 Aug;137:12-21. doi: 10.1016/j.jinorgbio.2014.04.001. Epub 2014 Apr 13. J Inorg Biochem. 2014. PMID: 24798373
-
Stabilization of G-quadruplex DNA, inhibition of telomerase activity, and tumor cell apoptosis by organoplatinum(II) complexes with oxoisoaporphine.J Med Chem. 2015 Mar 12;58(5):2159-79. doi: 10.1021/jm5012484. Epub 2015 Feb 18. J Med Chem. 2015. PMID: 25650792
-
Interactions of terpyridines and their Pt(II) complexes with G-quadruplex DNAs and telomerase inhibition.Int J Biol Macromol. 2013 Jun;57:1-8. doi: 10.1016/j.ijbiomac.2013.02.010. Epub 2013 Feb 19. Int J Biol Macromol. 2013. PMID: 23434432
-
Structure-based design of platinum(II) complexes as c-myc oncogene down-regulators and luminescent probes for G-quadruplex DNA.Chemistry. 2010 Jun 18;16(23):6900-11. doi: 10.1002/chem.201000167. Chemistry. 2010. PMID: 20437426
Cited by
-
Role of Telomeres and Telomeric Proteins in Human Malignancies and Their Therapeutic Potential.Cancers (Basel). 2020 Jul 14;12(7):1901. doi: 10.3390/cancers12071901. Cancers (Basel). 2020. PMID: 32674474 Free PMC article. Review.
-
Selectivity of Terpyridine Platinum Anticancer Drugs for G-quadruplex DNA.Molecules. 2019 Jan 23;24(3):404. doi: 10.3390/molecules24030404. Molecules. 2019. PMID: 30678027 Free PMC article.
-
Gaining Insights on the Interactions of a Class of Decorated (2-([2,2'-Bipyridin]-6-yl)phenyl)platinum Compounds with c-Myc Oncogene Promoter G-Quadruplex and Other DNA Structures.Chemistry. 2022 Sep 27;28(54):e202201497. doi: 10.1002/chem.202201497. Epub 2022 Aug 1. Chemistry. 2022. PMID: 35726630 Free PMC article.
-
Importance of Chiral Recognition in Designing Metal-Free Ligands for G-Quadruplex DNA.Molecules. 2019 Apr 15;24(8):1473. doi: 10.3390/molecules24081473. Molecules. 2019. PMID: 30991655 Free PMC article.
-
Natural aporphine alkaloids: A comprehensive review of phytochemistry, pharmacokinetics, anticancer activities, and clinical application.J Adv Res. 2024 Sep;63:231-253. doi: 10.1016/j.jare.2023.11.003. Epub 2023 Nov 5. J Adv Res. 2024. PMID: 37935346 Free PMC article. Review.
References
-
- Xu Y. Chemistry in human telomere biology: structure, function and targeting of telomere DNA/RNA. Chem Soc Rev. 2011;40:2719–2740. - PubMed
-
- Han H, Hurley LH. G-Quadruplex DNA: a potential target for anti-cancer drug design. Trends Pharmacol Sci. 2000;21:136–142. - PubMed
-
- Amrane S, Kerkour A, Bedrat A, Vialet B, Andreola ML, Mergny JL. Topology of a DNA G-qudruplex structure formed in the HIV-1 promoter: a potential target for anti-HIV drug development. J Am Chem Soc. 2014;136:5249–5252. - PubMed
-
- Paritala H, Firestine SM. Characterization of insulin ILPR sequences for their ability to adopt a G-quadruplex structure. Nucleos Nucleot Nucl. 2010;29:81–90. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

