Improving radiotherapy in cancer treatment: Promises and challenges
- PMID: 28977985
- PMCID: PMC5617545
- DOI: 10.18632/oncotarget.18409
Improving radiotherapy in cancer treatment: Promises and challenges
Abstract
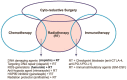
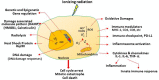
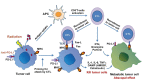
Effective radiotherapy for cancer has relied on the promise of maximally eradicating tumor cells while minimally killing normal cells. Technological advancement has provided state-of-the-art instrumentation that enables delivery of radiotherapy with great precision to tumor lesions with substantial reduced injury to normal tissues. Moreover, better understanding of radiobiology, particularly the mechanisms of radiation sensitivity and resistance in tumor lesions and toxicity in normal tissues, has improved the treatment efficacy of radiotherapy. Previous mechanism-based studies have identified many cellular targets that can affect radiation sensitivity, notably reactive oxygen species, DNA-damaging response signals, and tumor microenvironments. Several radiation sensitizers and protectors have been developed and clinically evaluated; however, many of these results are inconclusive, indicating that improvement remains needed. In this era of personalized medicine in which patients' genetic variations, transcriptome and proteomics, tumor metabolism and microenvironment, and tumor immunity are available. These new developments have provided opportunity for new target discovery. Several radiotherapy sensitivity-associated "gene signatures" have been reported although clinical validations are needed. Recently, several immune modifiers have been shown to associate with improved radiotherapy in preclinical models and in early clinical trials. Combination of radiotherapy and immunocheckpoint blockade has shown promising results especially in targeting metastatic tumors through abscopal response. In this article, we succinctly review recent advancements in the areas of mechanism-driven targets and exploitation of new targets from current radio-oncogenomic and radiation-immunotherapeutic approaches that bear clinical implications for improving the treatment efficacy of radiotherapy.
Keywords: DNA damage response; cancer genomics; hypoxia; immune check points; radiotherapy.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

