Microtubule inhibitor, SP-6-27 inhibits angiogenesis and induces apoptosis in ovarian cancer cells
- PMID: 28978013
- PMCID: PMC5620153
- DOI: 10.18632/oncotarget.17549
Microtubule inhibitor, SP-6-27 inhibits angiogenesis and induces apoptosis in ovarian cancer cells
Abstract
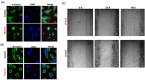
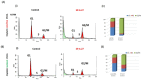
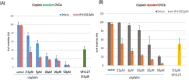
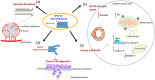
In ovarian cancer (OVCA), treatment failure due to chemo-resistance is a serious challenge. It is therefore critical to identify new therapies that are effective against resistant tumors and have reduced side effects. We recently identified 4-H-chromenes as tubulin depolymerizing agents that bind to colchicine site of beta-tubulin. Here, we screened a chemical library of substituted 4-H-chromenes and identified SP-6-27 to exhibit most potent anti-proliferative activity towards a panel of human cisplatin sensitive and resistant OVCA cell lines with 50% inhibitory concentration (IC50; mean ± SD) ranging from 0.10 ± 0.01 to 0.84 ± 0.20 μM. SP-6-27 exhibited minimum cytotoxicity to normal ovarian epithelia. A pronounced decrease in microtubule density as well as G2/M cell cycle arrest was observed in SP-6-27 treated cisplatin sensitive/resistant OVCA cells. The molecular mechanism of SP-6-27 induced cell death revealed modulation in cell-cycle regulation by upregulation of growth arrest and DNA damage inducible alpha transcripts (GADD45). An enhanced intrinsic apoptosis was observed in OVCA cells through upregulation of Bax, Apaf-1, caspase-6, -9, and caspase-3. In vitro wound healing assay revealed reduced OVCA cell migration upon SP-6-27 treatment. Additionally, SP-6-27 and cisplatin combinatorial treatment showed enhanced cytotoxicity in chemo-sensitive/resistant OVCA cells. Besides effect on cancer cells, SP-6-27 further restrained angiogenesis by inhibiting capillary tube formation by human umbilical vein endothelial cells (HUVEC). Together, these findings show that the chromene analog SP-6-27 is a novel chemotherapeutic agent that offers important advantages for the treatment of ovarian cancer.
Keywords: chromene; cisplatin resistance; microtubule inhibitor; ovarian cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflict of interest.
Figures



Similar articles
-
Expression of p53 in cisplatin-resistant ovarian cancer cell lines: modulation with the novel platinum analogue (1R, 2R-diaminocyclohexane)(trans-diacetato)(dichloro)-platinum(IV).Clin Cancer Res. 1999 Mar;5(3):655-63. Clin Cancer Res. 1999. PMID: 10100719
-
Profiling follicle stimulating hormone-induced gene expression changes in normal and malignant human ovarian surface epithelial cells.Oncogene. 2003 Jul 3;22(27):4243-56. doi: 10.1038/sj.onc.1206437. Oncogene. 2003. PMID: 12833147
-
A novel synthetic compound exerts effective anti-tumour activity in vivo via the inhibition of tubulin polymerisation in A549 cells.Biochem Pharmacol. 2015 Sep 1;97(1):51-61. doi: 10.1016/j.bcp.2015.07.008. Epub 2015 Jul 23. Biochem Pharmacol. 2015. PMID: 26212540
-
Synthesis and biological evaluation of diarylthiazole derivatives as antimitotic and antivascular agents with potent antitumor activity.Bioorg Med Chem. 2015 Jul 1;23(13):3337-50. doi: 10.1016/j.bmc.2015.04.055. Epub 2015 Apr 24. Bioorg Med Chem. 2015. PMID: 25937236
-
Clinical Implication of Metformin in Relation to Diabetes Mellitus and Ovarian Cancer.Biomedicines. 2021 Aug 16;9(8):1020. doi: 10.3390/biomedicines9081020. Biomedicines. 2021. PMID: 34440224 Free PMC article. Review.
Cited by
-
Magnetically recoverable Fe3O4@chitosan@Ni2B: a bio-based catalyst for one-pot green and efficient synthesis of tetrahydrobenzo[b]pyrans.Nanoscale Adv. 2025 May 9;7(12):3701-3721. doi: 10.1039/d4na01020e. eCollection 2025 Jun 10. Nanoscale Adv. 2025. PMID: 40352460 Free PMC article.
-
Synthesis of New Chromene Derivatives Targeting Triple-Negative Breast Cancer Cells.Cancers (Basel). 2023 May 9;15(10):2682. doi: 10.3390/cancers15102682. Cancers (Basel). 2023. PMID: 37345018 Free PMC article.
-
ZLM-7 inhibits the occurrence and angiogenesis of breast cancer through miR-212-3p/Sp1/VEGFA signal axis.Mol Med. 2020 Nov 13;26(1):109. doi: 10.1186/s10020-020-00239-2. Mol Med. 2020. PMID: 33187481 Free PMC article.
-
Novel Indole-Tethered Chromene Derivatives: Synthesis, Cytotoxic Properties, and Key Computational Insights.Pharmaceuticals (Basel). 2023 Feb 22;16(3):333. doi: 10.3390/ph16030333. Pharmaceuticals (Basel). 2023. PMID: 36986433 Free PMC article.
-
Recyclable mesalamine-functionalized magnetic nanoparticles (mesalamine/GPTMS@SiO2@Fe3O4) for tandem Knoevenagel-Michael cyclocondensation: grinding technique for the synthesis of biologically active 2-amino-4H-benzo[b]pyran derivatives.RSC Adv. 2023 Nov 20;13(48):33566-33587. doi: 10.1039/d3ra06560j. eCollection 2023 Nov 16. RSC Adv. 2023. PMID: 38020042 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

