Isolation and detection of circulating tumour cells from metastatic melanoma patients using a slanted spiral microfluidic device
- PMID: 28978038
- PMCID: PMC5620178
- DOI: 10.18632/oncotarget.18641
Isolation and detection of circulating tumour cells from metastatic melanoma patients using a slanted spiral microfluidic device
Abstract
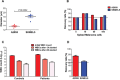
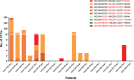
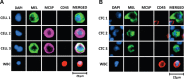
Circulating Tumour Cells (CTCs) are promising cancer biomarkers. Several methods have been developed to isolate CTCs from blood samples. However, the isolation of melanoma CTCs is very challenging as a result of their extraordinary heterogeneity, which has hindered their biological and clinical study. Thus, methods that isolate CTCs based on their physical properties, rather than surface marker expression, such as microfluidic devices, are greatly needed in melanoma. Here, we assessed the ability of the slanted spiral microfluidic device to isolate melanoma CTCs via label-free enrichment. We demonstrated that this device yields recovery rates of spiked melanoma cells of over 80% and 55%, after one or two rounds of enrichment, respectively. Concurrently, a two to three log reduction of white blood cells was achieved with one or two rounds of enrichment, respectively. We characterised the isolated CTCs using multimarker flow cytometry, immunocytochemistry and gene expression. The results demonstrated that CTCs from metastatic melanoma patients were highly heterogeneous and commonly expressed stem-like markers such as PAX3 and ABCB5. The implementation of the slanted microfluidic device for melanoma CTC isolation enables further understanding of the biology of melanoma metastasis for biomarker development and to inform future treatment approaches.
Keywords: circulating tumour cells (CTCs); metastatic melanoma; slanted spiral microfluidics.
Conflict of interest statement
CONFLICTS OF INTEREST All authors, except for M.H.F., declare no conflicts of interest. M.H.F. is inventor or co-inventor of US and international patents assigned to Brigham and Women’s Hospital and/or Boston Children's Hospital, Boston, MA, and licensed to Ticeba GmbH (Heidelberg, Germany) and Rheacell GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor to Ticeba GmbH and Rheacell GmbH & Co. KG. and participates in corporate sponsored research collaborations with Rheacell GmbH & Co. KG.
Figures

References
-
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. - DOI - PMC - PubMed
-
- Puzanov I, Amaravadi RK, McArthur GA, Flaherty KT, Chapman PB, Sosman JA, Ribas A, Shackleton M, Hwu P, Chmielowski B, Nolop KB, Lin PS, Kim KB. Long-term outcome in BRAF(V600E) melanoma patients treated with vemurafenib: patterns of disease progression and clinical management of limited progression. Eur J Cancer. 2015;51:1435–43. doi: 10.1016/j.ejca.2015.04.010. - DOI - PMC - PubMed
-
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of Ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94. doi: 10.1200/JCO.2014.56.2736. - DOI - PMC - PubMed
-
- Flaherty K, Davies MA, Grob JJ, Long GV, Nathan P, Ribas A, Robert C, Schadendorf D, Frederick DT, Hammond PT, Jane-Valbuena J, Mu XJ, Squires M, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) + trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. J Clin Oncol. 2016;34:9502.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

