GTSE1: a novel TEAD4-E2F1 target gene involved in cell protrusions formation in triple-negative breast cancer cell models
- PMID: 28978043
- PMCID: PMC5620183
- DOI: 10.18632/oncotarget.18691
GTSE1: a novel TEAD4-E2F1 target gene involved in cell protrusions formation in triple-negative breast cancer cell models
Abstract
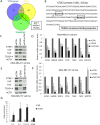
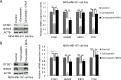
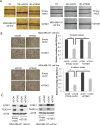
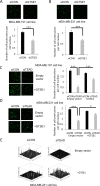
GTSE1 over-expression has been reported as a potential marker for metastasis in various types of malignancies, including breast cancer. Despite this, the transcriptional regulation of this protein and the causes of its misregulation in tumors remain largely unknown. The aims of this work were to elucidate how GTSE1 is regulated at the transcriptional level and to clarify the mechanism underlying GTSE1-dependent cell functions in triple-negative breast cancer (TNBC). Here, we identified GTSE1 as a novel target gene of the TEAD4 transcription factor, highlighting a role for the YAP and TAZ coactivators in the transcriptional regulation of GTSE1. Moreover, we found that TEAD4 controls the formation of cell protrusions required for cell migration through GTSE1, unveiling a relevant effector role for this protein in the TEAD-dependent cellular functions and confirming TEAD4 role in promoting invasion and metastasis in breast cancer. Finally, we highlighted a role for the pRb-E2F1 pathway in the control of GTSE1 transcription and observed that treatment with drugs targeting the pRb-E2F1 or YAP/TAZ-TEAD pathways dramatically downregulated the expression levels of GTSE1 and of other genes involved in the formation of metastasis, suggesting their potential use in the treatment of TNBC.
Keywords: E2F1; GTSE1; TEAD4; YAP/TAZ; cell protrusions.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have not conflicts of interest to declare.
Figures


Similar articles
-
Hippo/TEAD4 signaling pathway as a potential target for the treatment of breast cancer.Oncol Lett. 2021 Apr;21(4):313. doi: 10.3892/ol.2021.12574. Epub 2021 Feb 23. Oncol Lett. 2021. PMID: 33692845 Free PMC article. Review.
-
Structural and Functional Overview of TEAD4 in Cancer Biology.Onco Targets Ther. 2020 Oct 6;13:9865-9874. doi: 10.2147/OTT.S266649. eCollection 2020. Onco Targets Ther. 2020. PMID: 33116572 Free PMC article. Review.
-
TEAD4 as an Oncogene and a Mitochondrial Modulator.Front Cell Dev Biol. 2022 May 5;10:890419. doi: 10.3389/fcell.2022.890419. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35602596 Free PMC article. Review.
-
Tead and AP1 Coordinate Transcription and Motility.Cell Rep. 2016 Feb 9;14(5):1169-1180. doi: 10.1016/j.celrep.2015.12.104. Epub 2016 Jan 28. Cell Rep. 2016. PMID: 26832411 Free PMC article.
-
Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers.FEBS Open Bio. 2015 Jun 22;5:542-9. doi: 10.1016/j.fob.2015.06.007. eCollection 2015. FEBS Open Bio. 2015. PMID: 26199863 Free PMC article.
Cited by
-
GTSE1 is involved in breast cancer progression in p53 mutation-dependent manner.J Exp Clin Cancer Res. 2019 Apr 8;38(1):152. doi: 10.1186/s13046-019-1157-4. J Exp Clin Cancer Res. 2019. PMID: 30961661 Free PMC article.
-
Pan cancer characterization of genes whose expression has been associated with LINE-1 antisense promoter activity.Mob DNA. 2023 Sep 18;14(1):13. doi: 10.1186/s13100-023-00300-x. Mob DNA. 2023. PMID: 37723560 Free PMC article.
-
Molecular Mechanism of Hippo-YAP1/TAZ Pathway in Heart Development, Disease, and Regeneration.Front Physiol. 2020 Apr 23;11:389. doi: 10.3389/fphys.2020.00389. eCollection 2020. Front Physiol. 2020. PMID: 32390875 Free PMC article. Review.
-
Overexpression of TEAD4 correlates with poor prognosis of glioma and promotes cell invasion.Int J Clin Exp Pathol. 2018 Oct 1;11(10):4827-4835. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949557 Free PMC article.
-
Transcription profiling of feline mammary carcinomas and derived cell lines reveals biomarkers and drug targets associated with metabolic and cell cycle pathways.Sci Rep. 2022 Oct 11;12(1):17025. doi: 10.1038/s41598-022-20874-5. Sci Rep. 2022. PMID: 36220861 Free PMC article.
References
-
- Scolz M, Widlund PO, Piazza S, Bublik DR, Reber S, Peche LY, Ciani Y, Hubner N, Isokane M, Monte M, Ellenberg J, Hyman AA, Schneider C, et al. GTSE1 Is a microtubule plus-end tracking protein that regulates eb1-dependent cell migration. PLoS One. 2012;7:e51259. doi: 10.1371/journal.pone.0051259. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

