Native glycan fragments detected by MALDI-FT-ICR mass spectrometry imaging impact gastric cancer biology and patient outcome
- PMID: 28978092
- PMCID: PMC5620232
- DOI: 10.18632/oncotarget.19137
Native glycan fragments detected by MALDI-FT-ICR mass spectrometry imaging impact gastric cancer biology and patient outcome
Abstract
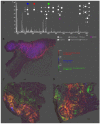
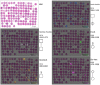
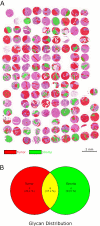
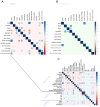
Glycosylation in cancer is a highly dynamic process that has a significant impact on tumor biology. Further, the attachment of aberrant glycan forms is already considered a hallmark of the disease state. Mass spectrometry has become a prominent approach to analyzing glycoconjugates. Specifically, matrix-assisted laser desorption/ionisation -mass spectrometric imaging (MALDI-MSI) is a powerful technique that combines mass spectrometry with histology and enables the spatially resolved and label-free detection of glycans. The most common approach to the analysis of glycans is the use of mass spectrometry adjunct to PNGase F digestion and other chemical reactions. In the current study, we perform the analysis of formalin-fixed, paraffin-embedded (FFPE) tissues for natively occurring bioactive glycan fragments without prior digestion or chemical reactions using MALDI-FT-ICR-MSI. We examined 106 primary resected gastric cancer patient tissues in a tissue microarray and correlated native-occurring fragments with clinical endpoints, therapeutic targets such as epidermal growth factor receptor (EGFR) and HER2/neu expressions and the proliferation marker MIB1. The detection of a glycosaminoglycan fragment in tumor stroma regions was determined to be an independent prognostic factor for gastric cancer patients. Native glycan fragments were significantly linked to the expression of EGFR, HER2/neu and MIB1. In conclusion, we are the first to report the in situ detection of native-occurring bioactive glycan fragments in FFPE tissues that influence patient outcomes. These findings highlight the significance of glycan fragments in gastric cancer tumor biology and patient outcome.
Keywords: MALDI; formalin-fixed paraffin-embedded tissue; gastric cancer; glycans; mass spectrometry imaging.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Hakomori S, Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983;71:231–51. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

