Pyruvate kinase M2 deregulation enhances the metastatic potential of tongue squamous cell carcinoma
- PMID: 28978113
- PMCID: PMC5620253
- DOI: 10.18632/oncotarget.19291
Pyruvate kinase M2 deregulation enhances the metastatic potential of tongue squamous cell carcinoma
Abstract
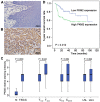
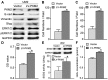
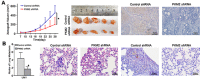
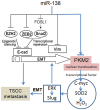
Pyruvate kinase M2 (PKM2) has been verified to correlate with the prognosis of many types of cancer. However, its role in the development and metastasis of tongue squamous cell carcinoma (TSCC) remains unclear. The immunohistochemistry (IHC) results confirmed that PKM2 is overexpressed in patients with TSCC. PKM2 up-regulation was related to lymph node metastasis and associated with reduced overall survival. According to the microarray analysis and Western blots, PKM2 expression was up-regulated in TSCC cells with enhanced metastatic potential. PKM2 knockdown inhibited cell migration and invasion, reduced SOD2 (manganese superoxide dismutase) activity and the intracellular H2O2 level, and inhibited tumour growth and lung metastasis in vivo. PKM2 overexpression promoted cell migration and invasion, and increased SOD2 activity and the intracellular H2O2 level. Moreover, miR-138 directly targeted PKM2 and inhibited PKM2 expression. Thus, PKM2 deregulation plays an important role in TSCC and may serve as a biomarker of metastatic potential or as a therapeutic target in patients with TSCC. PKM2, a miR-138 target gene, enhances the metastatic potential of TSCC through the SOD2-H2O2 pathway.
Keywords: manganese superoxide dismutase; metastasis; miR-138; pyruvate kinase M2; tongue squamous cell carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to declare.
Figures


Similar articles
-
High glucose enhances the metastatic potential of tongue squamous cell carcinoma via the PKM2 pathway.Oncotarget. 2017 Dec 4;8(67):111770-111779. doi: 10.18632/oncotarget.22907. eCollection 2017 Dec 19. Oncotarget. 2017. PMID: 29340090 Free PMC article.
-
Hexokinase 2 enhances the metastatic potential of tongue squamous cell carcinoma via the SOD2-H2O2 pathway.Oncotarget. 2017 Jan 10;8(2):3344-3354. doi: 10.18632/oncotarget.13763. Oncotarget. 2017. PMID: 27926482 Free PMC article.
-
Deregulation of manganese superoxide dismutase (SOD2) expression and lymph node metastasis in tongue squamous cell carcinoma.BMC Cancer. 2010 Jul 9;10:365. doi: 10.1186/1471-2407-10-365. BMC Cancer. 2010. PMID: 20618948 Free PMC article.
-
Bmi1 drives stem-like properties and is associated with migration, invasion, and poor prognosis in tongue squamous cell carcinoma.Int J Biol Sci. 2015 Jan 1;11(1):1-10. doi: 10.7150/ijbs.10405. eCollection 2015. Int J Biol Sci. 2015. PMID: 25552924 Free PMC article.
-
Overexpression of metabolic markers PKM2 and LDH5 correlates with aggressive clinicopathological features and adverse patient prognosis in tongue cancer.Histopathology. 2014 Nov;65(5):595-605. doi: 10.1111/his.12441. Epub 2014 Sep 2. Histopathology. 2014. PMID: 24762230
Cited by
-
Identification of Prognostic Markers for Head and NeckSquamous Cell Carcinoma Based on Glycolysis-Related Genes.Evid Based Complement Alternat Med. 2022 Jul 7;2022:2762595. doi: 10.1155/2022/2762595. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35845594 Free PMC article.
-
Immunohistochemical detection of MnSOD in colon adenocarcinoma patients - clinical application.Prz Gastroenterol. 2024;19(2):186-193. doi: 10.5114/pg.2024.139238. Epub 2024 Apr 26. Prz Gastroenterol. 2024. PMID: 38939067 Free PMC article.
-
The interplay between non-coding RNAs and alternative splicing: from regulatory mechanism to therapeutic implications in cancer.Theranostics. 2023 Apr 23;13(8):2616-2631. doi: 10.7150/thno.83920. eCollection 2023. Theranostics. 2023. PMID: 37215575 Free PMC article. Review.
-
High glucose enhances the metastatic potential of tongue squamous cell carcinoma via the PKM2 pathway.Oncotarget. 2017 Dec 4;8(67):111770-111779. doi: 10.18632/oncotarget.22907. eCollection 2017 Dec 19. Oncotarget. 2017. PMID: 29340090 Free PMC article.
-
Strong SOD2 expression and HPV-16/18 positivity are independent events in cervical cancer.Oncotarget. 2018 Apr 24;9(31):21630-21640. doi: 10.18632/oncotarget.24850. eCollection 2018 Apr 24. Oncotarget. 2018. PMID: 29774090 Free PMC article.
References
-
- Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res. 2012;18:5554–5561. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

