A cerebrospinal fluid microRNA signature as biomarker for glioblastoma
- PMID: 28978155
- PMCID: PMC5620295
- DOI: 10.18632/oncotarget.18332
A cerebrospinal fluid microRNA signature as biomarker for glioblastoma
Abstract
Purpose: To develop a cerebrospinal fluid (CSF) miRNA diagnostic biomarker for glioblastoma.
Experimental design: Glioblastoma tissue and matched CSF from the same patient (obtained prior to tumor manipulation) were profiled by TaqMan OpenArray® Human MicroRNA Panel. CSF miRNA profiles from glioblastoma patients and controls were created from three discovery cohorts and confirmed in two validation cohorts.
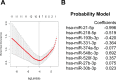
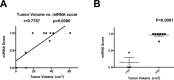
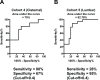
Results: miRNA profiles from clinical CSF correlated with those found in glioblastoma tissues. Comparison of CSF miRNA profiles between glioblastoma patients and non-brain tumor patients yielded a tumor "signature" consisting of nine miRNAs. The "signature" correlated with glioblastoma tumor volume (p=0.008). When prospectively applied to cisternal CSF, the sensitivity and specificity of the 'signature' for glioblastoma detection were 67% and 80%, respectively. For lumbar CSF, the sensitivity and specificity of the signature were 28% and 95%, respectively. Comparable results were obtained from analyses of CSF extracellular vesicles (EVs) and crude CSF.
Conclusion: We report a CSF miRNA signature as a "liquid biopsy" diagnostic platform for glioblastoma.
Keywords: CSF; extracellular vesicle; liquid biopsy.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there is no conflict of interest.
Figures


References
-
- Bartek J, Jr, Ng K, Bartek J, Fischer W, Carter B, Chen CC. Key concepts in glioblastoma therapy. J Neurol Neurosurg Psychiatry. 2012;83:753–60. https://doi.org/10.1136/jnnp-2011-300709 - DOI - PubMed
-
- Ng K, Kim R, Kesari S, Carter B, Chen CC. Genomic profiling of glioblastoma: convergence of fundamental biologic tenets and novel insights. J Neurooncol. 2012;107:1–12. - PubMed
-
- Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 2003;145:5–10. - PubMed
-
- Air EL, Leach JL, Warnick RE, McPherson CM. Comparing the risks of frameless stereotactic biopsy in eloquent and noneloquent regions of the brain: a retrospective review of 284 cases. J Neurosurg. 2009;111:820–24. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

