Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies
- PMID: 28978184
- PMCID: PMC5620324
- DOI: 10.18632/oncotarget.19079
Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies
Abstract
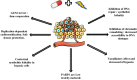
Background: Poly-(ADP-Ribose)-Polymerase (PARP) inhibitors are becoming important actors of anti-neoplasic agents landscape, with recent but narrow FDA's approvals for ovarian BRCA mutated cancers and prostatic cancer. Nevertheless, PARP inhibitors are also promising drugs for combined treatments particularly with radiotherapy. More than seven PARP inhibitors have been currently developed. Central Role of PARP in DNA repair, makes consider PARP inhibitor as potential radiosensitizers, especially for tumors with DNA repair defects, such as BRCA mutation, because of synthetic lethality. Furthermore the replication-dependent activity of PARP inhibitor helps to maintain the differential effect between tumoral and healthy tissues. Inhibition of chromatin remodeling, G2/M arrest, vasodilatory effect induced by PARP inhibitor, also participate to their radio-sensitization effect.
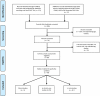
Materials and methods: Here, after highlighting mechanisms of PARP inhibitors radiosensitization we methodically searched PubMed, Google Scholar, Cochrane Databases and meeting proceedings for human pre-clinical and clinical studies that evaluated PARP inhibitor radiosensitizing effect. Enhancement ratio, when available, was systematically reported.
Results: Sixty four studies finally met our selection criteria and were included in the analysis. Only three pre-clinical studies didn't find any radiosensitizing effect. Median enhancement ratio vary from 1,3 for prostate tumors to 1,5 for lung cancers. Nine phase I or II trials assessed safety data.
Conclusion: PARP inhibitors are promising radiosensitizers, but need more clinical investigation. The next ten years will be determining for judging their real potential.
Keywords: poly(ADP-ribose)-polymerase inhibitors; radiobiology; radiosensitization; radiotherapy.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures

References
-
- Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NMB, Jackson SP, Smith GCM, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. https://doi.org/10.1038/nature03445 - DOI - PubMed
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. https://doi.org/10.1038/nature03443 - DOI - PubMed
-
- Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–62. https://doi.org/10.1016/j.ijrobp.2013.07.022 - DOI - PMC - PubMed
-
- Mansour WY, Rhein T, Dahm-Daphi J. The alternative end-joining pathway for repair of DNA double-strand breaks requires PARP1 but is not dependent upon microhomologies. Nucleic Acids Res. 2010;38:6065–77. https://doi.org/10.1093/nar/gkq387 - DOI - PMC - PubMed
-
- Schultz N, Lopez E, Saleh-Gohari N, Helleday T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–64. https://doi.org/10.1093/nar/gkg703 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

