The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis
- PMID: 28978186
- PMCID: PMC5620326
- DOI: 10.18632/oncotarget.19932
The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis
Abstract
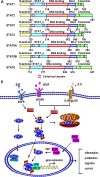
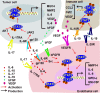
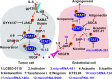
Angiogenesis is the development of new blood vessels, which is required for tumor growth and metastasis. Signal transducer and activator of transcription factor 3 (STAT3) is a transcription factor that regulates a variety of cellular events including proliferation, differentiation and apoptosis. Previous studies revealed that activation of STAT3 promotes tumor angiogenesis. In this review, we described the activities of STAT3 signaling in different cell types involved in angiogenesis. Particularly, we elucidated the molecular mechanisms of STAT3-mediated gene regulation in angiogenic endothelial cells in response to external stimulations such as hypoxia and inflammation. The potential for STAT3 as a therapeutic target was also discussed. Overall, this review provides mechanistic insights for the roles of STAT3 signaling in tumor angiogenesis.
Keywords: STAT3; endothelial cells; transcriptional regulation; tumor angiogenesis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


Similar articles
-
Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment.Arch Pharm Res. 2016 Aug;39(8):1085-99. doi: 10.1007/s12272-016-0795-8. Epub 2016 Aug 11. Arch Pharm Res. 2016. PMID: 27515050 Review.
-
IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer.Tumour Biol. 2016 Apr;37(4):5493-501. doi: 10.1007/s13277-015-4372-4. Epub 2015 Nov 13. Tumour Biol. 2016. PMID: 26566627
-
STAT3: a critical transcription activator in angiogenesis.Med Res Rev. 2008 Mar;28(2):185-200. doi: 10.1002/med.20101. Med Res Rev. 2008. PMID: 17457812 Review.
-
NF-YA promotes invasion and angiogenesis by upregulating EZH2-STAT3 signaling in human melanoma cells.Oncol Rep. 2016 Jun;35(6):3630-8. doi: 10.3892/or.2016.4761. Epub 2016 Apr 20. Oncol Rep. 2016. PMID: 27109360
-
Identification of NCK1 as a novel downstream effector of STAT3 in colorectal cancer metastasis and angiogenesis.Cell Signal. 2017 Aug;36:67-78. doi: 10.1016/j.cellsig.2017.04.020. Epub 2017 Apr 25. Cell Signal. 2017. PMID: 28455144
Cited by
-
JAK2/STAT3 pathway mediates neuroprotective and pro-angiogenic treatment effects of adult human neural stem cells in middle cerebral artery occlusion stroke animal models.Aging (Albany NY). 2022 Nov 29;14(22):8944-8969. doi: 10.18632/aging.204410. Epub 2022 Nov 29. Aging (Albany NY). 2022. PMID: 36446389 Free PMC article.
-
STAT6 Upregulates NRP1 Expression in Endothelial Cells and Promotes Angiogenesis.Front Oncol. 2022 May 5;12:823377. doi: 10.3389/fonc.2022.823377. eCollection 2022. Front Oncol. 2022. PMID: 35600336 Free PMC article.
-
Exosomes released by oxidative stress-induced mesenchymal stem cells promote murine mammary tumor progression through activating the STAT3 signaling pathway.Mol Cell Biochem. 2024 Dec;479(12):3375-3391. doi: 10.1007/s11010-024-04934-0. Epub 2024 Feb 13. Mol Cell Biochem. 2024. PMID: 38349465
-
Effect of CLIP3 Upregulation on Astrocyte Proliferation and Subsequent Glial Scar Formation in the Rat Spinal Cord via STAT3 Pathway After Injury.J Mol Neurosci. 2018 Jan;64(1):117-128. doi: 10.1007/s12031-017-0998-6. Epub 2017 Dec 7. J Mol Neurosci. 2018. PMID: 29218499
-
Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19.Biomed Pharmacother. 2020 Dec;132:110886. doi: 10.1016/j.biopha.2020.110886. Epub 2020 Oct 16. Biomed Pharmacother. 2020. PMID: 33113418 Free PMC article. Review.
References
-
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307:C25–38. https://doi.org/10.1152/ajpcell.00084.2014 - DOI - PMC - PubMed
-
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. https://doi.org/10.1042/cs20140278 - DOI - PMC - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. https://doi.org/10.1038/nrd2115 - DOI - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. https://doi.org/10.1038/nm0603-653 - DOI - PubMed
-
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. https://doi.org/10.1038/nm.2537 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

