Effects of action observation therapy and mirror therapy after stroke on rehabilitation outcomes and neural mechanisms by MEG: study protocol for a randomized controlled trial
- PMID: 28978349
- PMCID: PMC5628464
- DOI: 10.1186/s13063-017-2205-z
Effects of action observation therapy and mirror therapy after stroke on rehabilitation outcomes and neural mechanisms by MEG: study protocol for a randomized controlled trial
Abstract
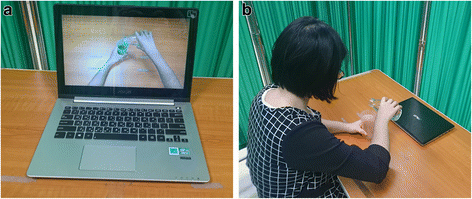
Background: Loss of upper-extremity motor function is one of the most debilitating deficits following stroke. Two promising treatment approaches, action observation therapy (AOT) and mirror therapy (MT), aim to enhance motor learning and promote neural reorganization in patients through different afferent inputs and patterns of visual feedback. Both approaches involve different patterns of motor observation, imitation, and execution but share some similar neural bases of the mirror neuron system. AOT and MT used in stroke rehabilitation may confer differential benefits and neural activities that remain to be determined. This clinical trial aims to investigate and compare treatment effects and neural activity changes of AOT and MT with those of the control intervention in patients with subacute stroke.
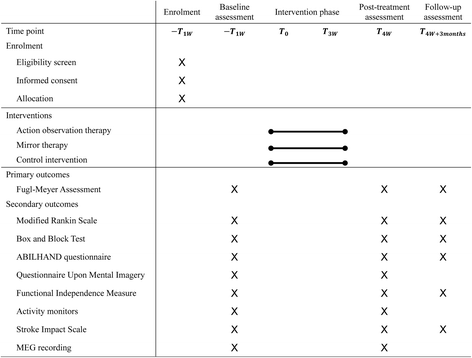
Methods/design: An estimated total of 90 patients with subacute stroke will be recruited for this study. All participants will be randomly assigned to receive AOT, MT, or control intervention for a 3-week training period (15 sessions). Outcome measurements will be taken at baseline, immediately after treatment, and at the 3-month follow-up. For the magnetoencephalography (MEG) study, we anticipate that we will recruit 12 to 15 patients per group. The primary outcome will be the Fugl-Meyer Assessment score. Secondary outcomes will include the modified Rankin Scale, the Box and Block Test, the ABILHAND questionnaire, the Questionnaire Upon Mental Imagery, the Functional Independence Measure, activity monitors, the Stroke Impact Scale version 3.0, and MEG signals.
Discussion: This clinical trial will provide scientific evidence of treatment effects on motor, functional outcomes, and neural activity mechanisms after AOT and MT in patients with subacute stroke. Further application and use of AOT and MT may include telerehabilitation or home-based rehabilitation through web-based or video teaching.
Trial registration: ClinicalTrials.gov, ID: NCT02871700 . Registered on 1 August 2016.
Keywords: Action observation; Magnetoencephalography; Mirror therapy; Neurorehabilitation; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
This study has been approved by the Institutional Review Board of Chang Gung Medical Foundation (Approval No. 104-9173A3) in Taiwan. The study will be conducted in accordance with the Helsinki Declaration and the International Conference on Harmonization Guidelines for Good Clinical Practice. All participating therapists and evaluators will be informed of their responsibilities. Information about the benefits and potential risks of participation will be described in the Informed Consent Form.
Consent for publication
Volunteer consent was provided for the photographs in Figs. 2, 3, and 4 to be included in the present study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011;25:838–46. doi: 10.1177/1545968311413906. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical