Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems
- PMID: 28978350
- PMCID: PMC5628487
- DOI: 10.1186/s40168-017-0348-5
Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems
Abstract
Background: Several biotic and abiotic factors have been reported to influence the proliferation of microbes, including Legionella pneumophila, in hot water premise plumbing systems, but their combined effects have not been systematically evaluated. Here, we utilize simulated household water heaters to examine the effects of stepwise increases in temperature (32-53 °C), pipe material (copper vs. cross-linked polyethylene (PEX)), and influent assimilable organic carbon (0-700 μg/L) on opportunistic pathogen gene copy numbers and the microbiota composition, as determined by quantitative polymerase chain reaction and 16S rRNA gene amplicon sequencing.
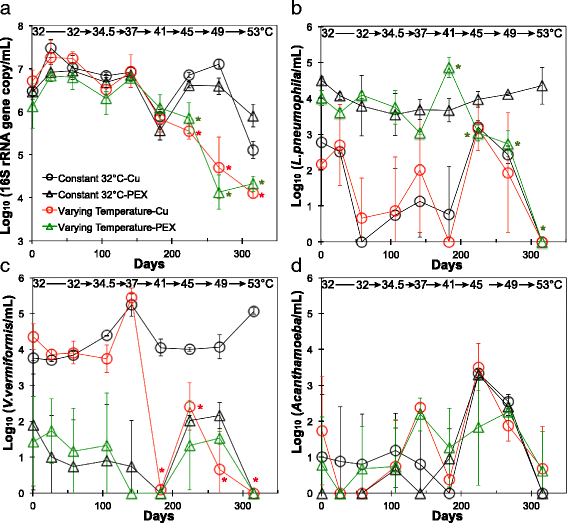
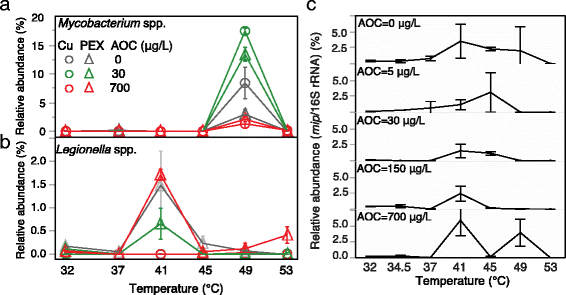
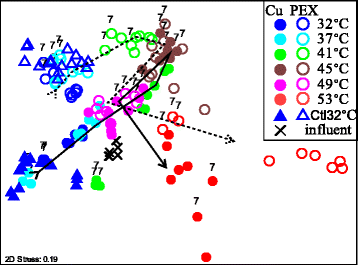
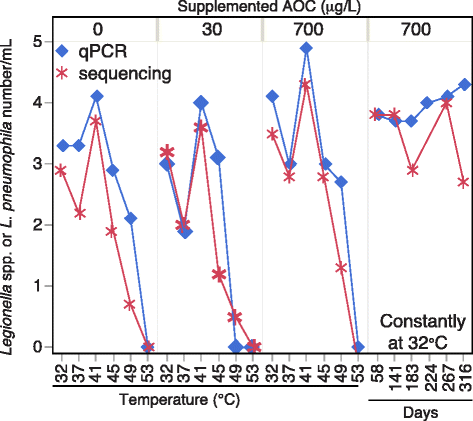
Results: Temperature had an overarching influence on both the microbiota composition and L. pneumophila numbers. L. pneumophila peaked at 41 °C in the presence of PEX (1.58 × 105 gene copies/mL). At 53 °C, L. pneumophila was not detected. Several operational taxonomic units (OTUs) persisted across all conditions, accounting for 50% of the microbiota composition from 32 to 49 °C and 20% at 53 °C. Pipe material most strongly influenced microbiota composition at lower temperatures, driven by five to six OTUs enriched with each material. Copper pipes supported less L. pneumophila than PEX pipes (mean 2.5 log10 lower) at temperatures ≤ 41 °C, but showed no difference in total bacterial numbers. Differences between pipe materials diminished with elevated temperature, probably resulting from decreased release of copper ions. At temperatures ≤ 45 °C, influent assimilable organic carbon correlated well with total bacterial numbers, but not with L. pneumophila numbers. At 53 °C, PEX pipes leached organic carbon, reducing the importance of dosed organic carbon. L. pneumophila numbers correlated with a Legionella OTU and a Methylophilus OTU identified by amplicon sequencing.
Conclusions: Temperature was the most effective factor for the control of L. pneumophila, while microbiota composition shifted with each stepwise temperature increase. While copper pipe may also help shape the microbiota composition and limit L. pneumophila proliferation, its benefits might be constrained at higher temperatures. Influent assimilable organic carbon affected total bacterial numbers, but had minimal influence on opportunistic pathogen gene numbers or microbiota composition. These findings provide guidance among multiple control measures for the growth of opportunistic pathogens in hot water plumbing and insight into the mediating role of microbial ecological factors.
Keywords: Hot water; Legionella; Opportunistic pathogen; Pipe material; Premise plumbing; Temperature control.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Replicable simulation of distal hot water premise plumbing using convectively-mixed pipe reactors.PLoS One. 2020 Sep 16;15(9):e0238385. doi: 10.1371/journal.pone.0238385. eCollection 2020. PLoS One. 2020. PMID: 32936810 Free PMC article.
-
Effect of heat shock on hot water plumbing microbiota and Legionella pneumophila control.Microbiome. 2018 Feb 9;6(1):30. doi: 10.1186/s40168-018-0406-7. Microbiome. 2018. PMID: 29426363 Free PMC article.
-
Water heater temperature set point and water use patterns influence Legionella pneumophila and associated microorganisms at the tap.Microbiome. 2015 Dec 1;3:67. doi: 10.1186/s40168-015-0134-1. Microbiome. 2015. PMID: 26627188 Free PMC article.
-
Critical Review: Propensity of Premise Plumbing Pipe Materials to Enhance or Diminish Growth of Legionella and Other Opportunistic Pathogens.Pathogens. 2020 Nov 17;9(11):957. doi: 10.3390/pathogens9110957. Pathogens. 2020. PMID: 33212943 Free PMC article. Review.
-
Efficacy of chlorine-based disinfectants to control Legionella within premise plumbing systems.Water Res. 2024 Aug 1;259:121794. doi: 10.1016/j.watres.2024.121794. Epub 2024 May 25. Water Res. 2024. PMID: 38824796
Cited by
-
Tenets of a holistic approach to drinking water-associated pathogen research, management, and communication.Water Res. 2022 Mar 1;211:117997. doi: 10.1016/j.watres.2021.117997. Epub 2021 Dec 22. Water Res. 2022. PMID: 34999316 Free PMC article. Review.
-
Retrospective Analysis of Drinking Water Microcosm Microbiomes Reveals an Apparent Antagonistic Relationship between and.Environ Sci Technol Lett. 2025 Jul 17;12(8):990-996. doi: 10.1021/acs.estlett.5c00590. eCollection 2025 Aug 12. Environ Sci Technol Lett. 2025. PMID: 40822855 Free PMC article.
-
Replicable simulation of distal hot water premise plumbing using convectively-mixed pipe reactors.PLoS One. 2020 Sep 16;15(9):e0238385. doi: 10.1371/journal.pone.0238385. eCollection 2020. PLoS One. 2020. PMID: 32936810 Free PMC article.
-
Understanding building-occupant-microbiome interactions toward healthy built environments: A review.Front Environ Sci Eng. 2021;15(4):65. doi: 10.1007/s11783-020-1357-3. Epub 2020 Oct 22. Front Environ Sci Eng. 2021. PMID: 33145119 Free PMC article. Review.
-
Electrically heatable carbon nanotube point-of-use filters for effective separation and in-situ inactivation of Legionella pneumophila.Chem Eng J. 2019;366:21-26. doi: 10.1016/j.cej.2019.02.054. Chem Eng J. 2019. PMID: 31275054 Free PMC article.
References
-
- Brunkard JM, Ailes E, Roberts VA, Hill V, Hilborn ED, Craun GF, et al. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2007–2008. MMWR Surveill Summ. 2011;60:38–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

