The CNS in inbred transgenic models of 4-repeat Tauopathy develops consistent tau seeding capacity yet focal and diverse patterns of protein deposition
- PMID: 28978354
- PMCID: PMC5628424
- DOI: 10.1186/s13024-017-0215-7
The CNS in inbred transgenic models of 4-repeat Tauopathy develops consistent tau seeding capacity yet focal and diverse patterns of protein deposition
Abstract
Background: MAPT mutations cause neurodegenerative diseases such as frontotemporal dementia but, strikingly, patients with the same mutation may have different clinical phenotypes.
Methods: Given heterogeneities observed in a transgenic (Tg) mouse line expressing low levels of human (2 N, 4R) P301L Tau, we backcrossed founder stocks of mice to C57BL/6Tac, 129/SvEvTac and FVB/NJ inbred backgrounds to discern the role of genetic versus environmental effects on disease-related phenotypes.
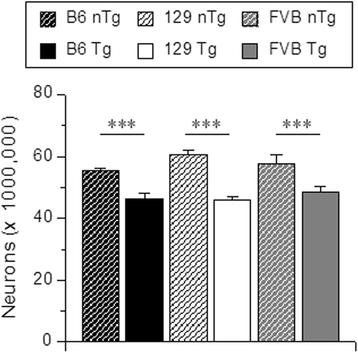
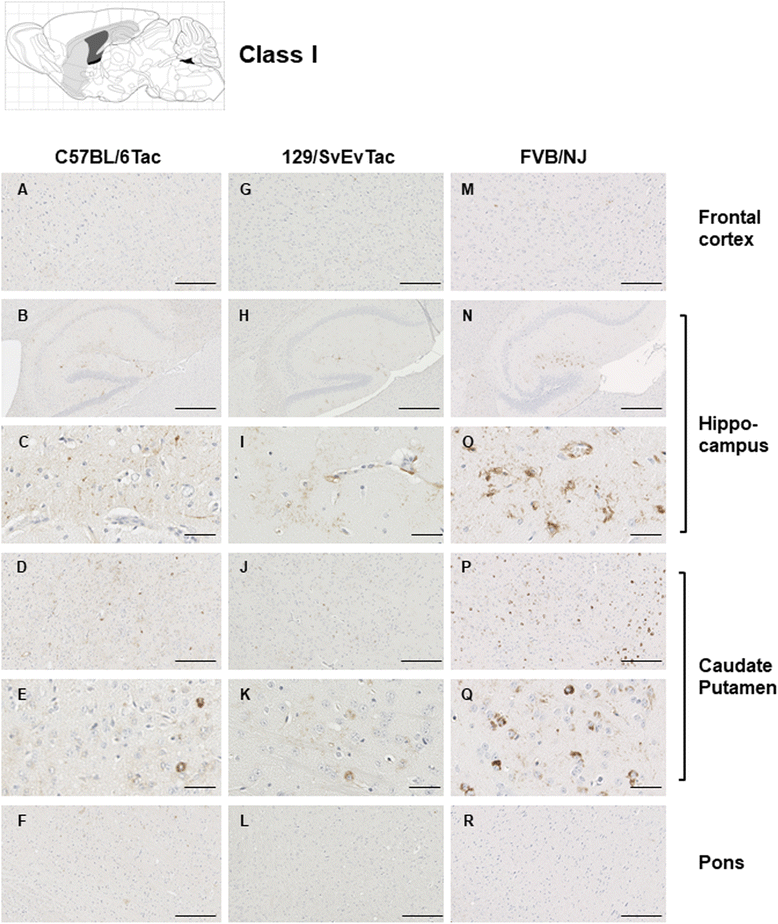
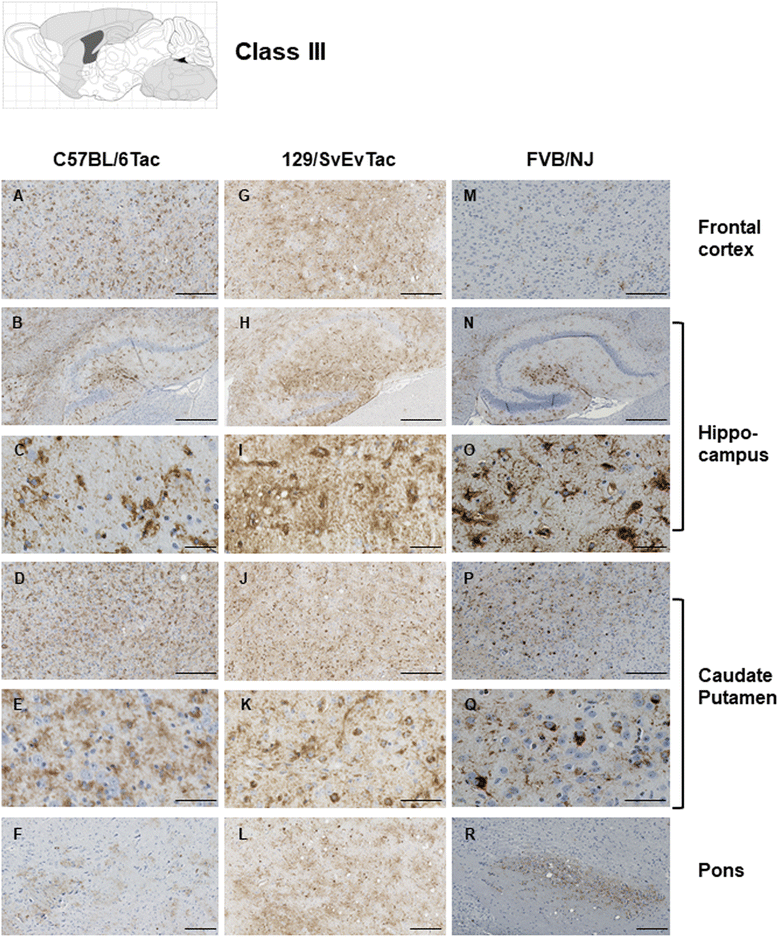
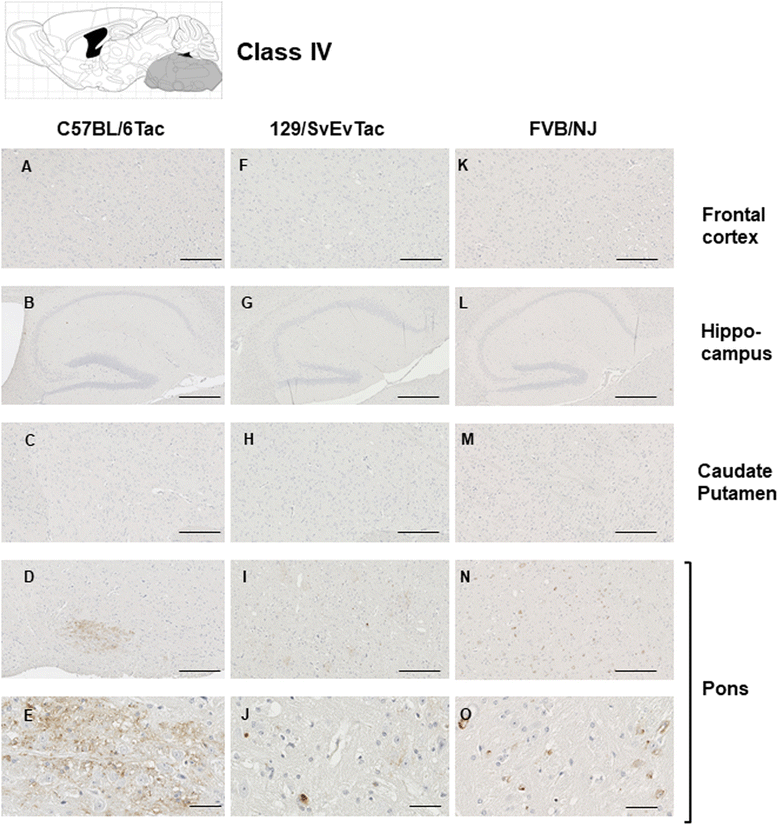
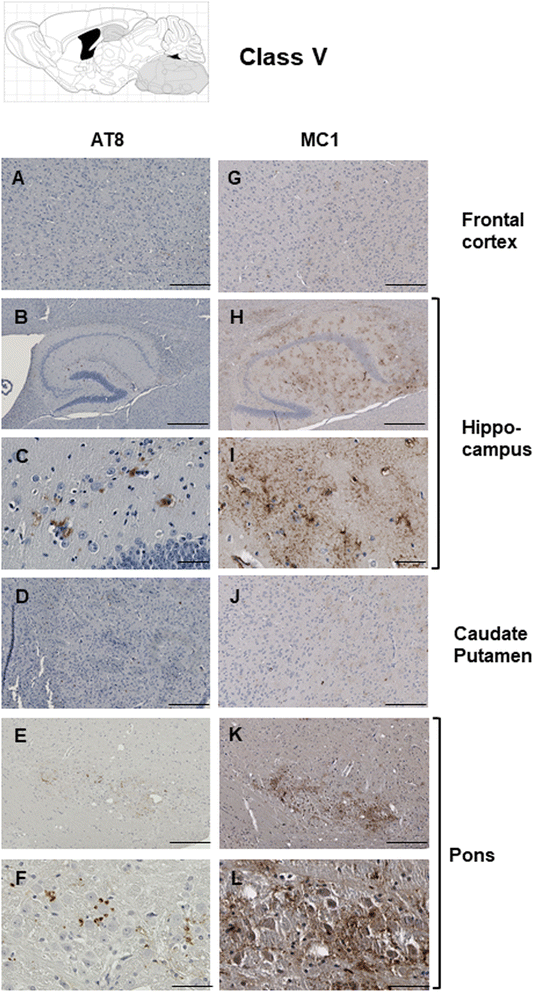
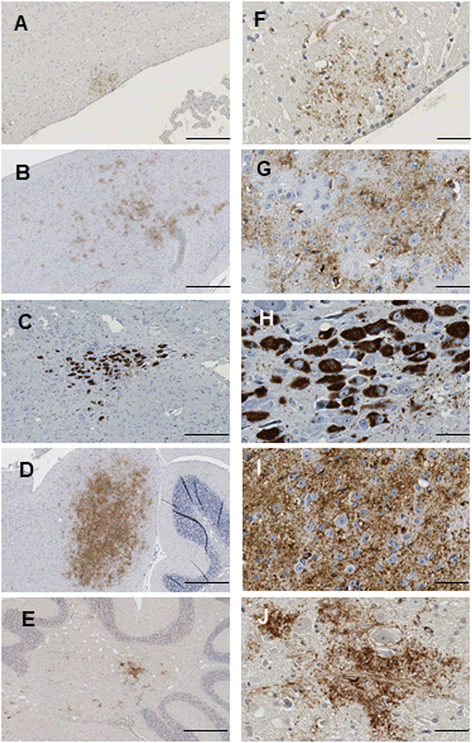
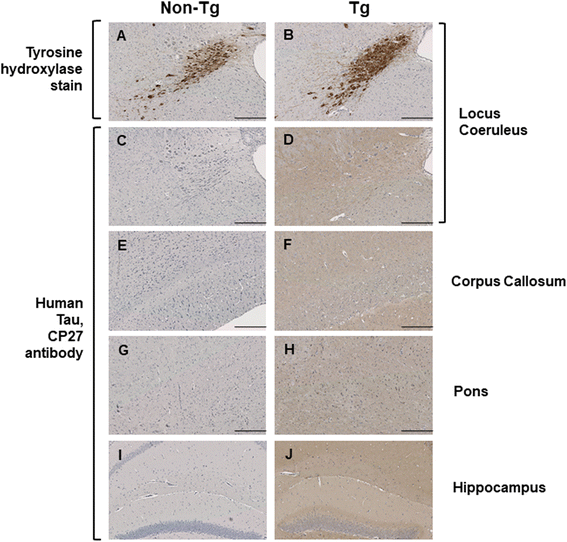
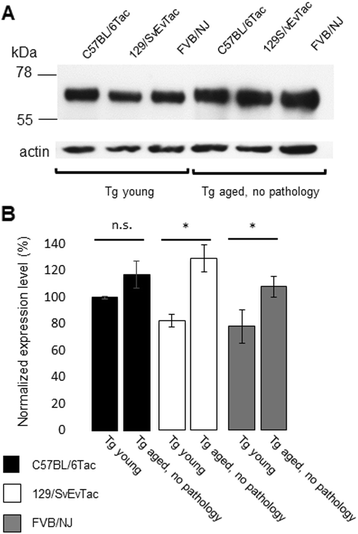
Results: Three inbred derivatives of a TgTauP301L founder line had similar quality and steady-state quantity of Tau production, accumulation of abnormally phosphorylated 64-68 kDa Tau species from 90 days of age onwards and neuronal loss in aged Tg mice. Variegation was not seen in the pattern of transgene expression and seeding properties in a fluorescence-based cellular assay indicated a single "strain" of misfolded Tau. However, in other regards, the aged Tg mice were heterogeneous; there was incomplete penetrance for Tau deposition despite maintained transgene expression in aged animals and, for animals with Tau deposits, distinctions were noted even within each subline. Three classes of rostral deposition in the cortex, hippocampus and striatum accounted for 75% of pathology-positive mice yet the mean ages of mice scored as class I, II or III were not significantly different and, hence, did not fit with a predictable progression from one class to another defined by chronological age. Two other patterns of Tau deposition designated as classes IV and V, occurred in caudal structures. Other pathology-positive Tg mice of similar age not falling within classes I-V presented with focal accumulations in additional caudal neuroanatomical areas including the locus coeruleus. Electron microscopy revealed that brains of Classes I, II and IV animals all exhibit straight filaments, but with coiled filaments and occasional twisted filaments apparent in Class I. Most strikingly, Class I, II and IV animals presented with distinct western blot signatures after trypsin digestion of sarkosyl-insoluble Tau.
Conclusions: Qualitative variations in the neuroanatomy of Tau deposition in genetically constrained slow models of primary Tauopathy establish that non-synchronous, focal events contribute to the pathogenic process. Phenotypic diversity in these models suggests a potential parallel to the phenotypic variation seen in P301L patients.
Keywords: Aging; Focal pathology; Neuronal loss; P301L mutation; Stochastic events; Transgenic mouse.
Conflict of interest statement
Ethics approval
All animal studies described herein were performed in accordance with Canadian Council on Animal Care (CCAC) guidelines, with specific protocols approved by the animal care use committee for Health Sciences Laboratory Animal Services at the University of Alberta (protocol AUP00000356).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







Similar articles
-
The olfactory epithelium: a critical gateway for pathological tau propagation and a target for mitigating tauopathy in the central nervous system.Acta Neuropathol. 2025 Jun 19;149(1):64. doi: 10.1007/s00401-025-02902-6. Acta Neuropathol. 2025. PMID: 40536690 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Small molecule inhibitor of tau self-association in a mouse model of tauopathy: A preventive study in P301L tau JNPL3 mice.PLoS One. 2023 Aug 9;18(8):e0286523. doi: 10.1371/journal.pone.0286523. eCollection 2023. PLoS One. 2023. PMID: 37556474 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
Cited by
-
Diverse, evolving conformer populations drive distinct phenotypes in frontotemporal lobar degeneration caused by the same MAPT-P301L mutation.Acta Neuropathol. 2020 Jun;139(6):1045-1070. doi: 10.1007/s00401-020-02148-4. Epub 2020 Mar 26. Acta Neuropathol. 2020. PMID: 32219515 Free PMC article.
-
Microbial Composition, Disease Trajectory and Genetic Background in a Slow Onset Model of Frontotemporal Lobar Degeneration.Biomolecules. 2025 Apr 29;15(5):636. doi: 10.3390/biom15050636. Biomolecules. 2025. PMID: 40427529 Free PMC article.
-
Cellular Biology of Tau Diversity and Pathogenic Conformers.Front Neurol. 2020 Nov 12;11:590199. doi: 10.3389/fneur.2020.590199. eCollection 2020. Front Neurol. 2020. PMID: 33304310 Free PMC article. Review.
-
Genetically modified non-human primate models for research on neurodegenerative diseases.Zool Res. 2024 Mar 18;45(2):263-274. doi: 10.24272/j.issn.2095-8137.2023.197. Zool Res. 2024. PMID: 38287907 Free PMC article. Review.
-
Seizures are a druggable mechanistic link between TBI and subsequent tauopathy.Elife. 2021 Feb 2;10:e58744. doi: 10.7554/eLife.58744. Elife. 2021. PMID: 33527898 Free PMC article.
References
-
- Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D'Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference Participants. Ann Neurol 1997;41(6):706–715. - PubMed
-
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

