pH Dependence of Charge Multipole Moments in Proteins
- PMID: 28978439
- PMCID: PMC5627345
- DOI: 10.1016/j.bpj.2017.08.017
pH Dependence of Charge Multipole Moments in Proteins
Abstract
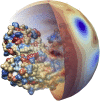
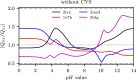
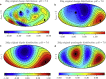
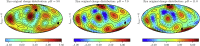
Electrostatic interactions play a fundamental role in the structure and function of proteins. Due to ionizable amino acid residues present on the solvent-exposed surfaces of proteins, the protein charge is not constant but varies with the changes in the environment-most notably, the pH of the surrounding solution. We study the effects of pH on the charge of four globular proteins by expanding their surface charge distributions in terms of multipoles. The detailed representation of the charges on the proteins is in this way replaced by the magnitudes and orientations of the multipole moments of varying order. Focusing on the three lowest-order multipoles-the total charge, dipole, and quadrupole moment-we show that the value of pH influences not only their magnitudes, but more notably and importantly also the spatial orientation of their principal axes. Our findings imply important consequences for the study of protein-protein interactions and the assembly of both proteinaceous shells and patchy colloids with dissociable charge groups.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
From discrete to continuous description of spherical surface charge distributions.Soft Matter. 2018 Feb 14;14(7):1149-1161. doi: 10.1039/c7sm02207g. Soft Matter. 2018. PMID: 29345714
-
Refolding of SDS-Unfolded Proteins by Nonionic Surfactants.Biophys J. 2017 Apr 25;112(8):1609-1620. doi: 10.1016/j.bpj.2017.03.013. Biophys J. 2017. PMID: 28445752 Free PMC article.
-
Measurements of the wettability of protein-covered hydroxyapatite surfaces.Caries Res. 1999 Nov-Dec;33(6):473-8. doi: 10.1159/000016554. Caries Res. 1999. PMID: 10529534
-
Analysis of protein solvent accessible surfaces by photochemical oxidation and mass spectrometry.Anal Chem. 2004 Feb 1;76(3):672-83. doi: 10.1021/ac0302004. Anal Chem. 2004. PMID: 14750862
-
Surfaces coated with protein layers: a surface force and ESCA study.Biomaterials. 1998 Mar;19(4-5):371-86. doi: 10.1016/s0142-9612(97)00114-2. Biomaterials. 1998. PMID: 9677151 Review.
Cited by
-
Integration of Cypoviruses into polyhedrin matrix.Nanoscale Adv. 2023 Jul 16;5(16):4140-4148. doi: 10.1039/d3na00393k. eCollection 2023 Aug 8. Nanoscale Adv. 2023. PMID: 37560430 Free PMC article.
-
Insights into the ZIKV NS1 Virology from Different Strains through a Fine Analysis of Physicochemical Properties.ACS Omega. 2018 Nov 29;3(11):16212-16229. doi: 10.1021/acsomega.8b02081. eCollection 2018 Nov 30. ACS Omega. 2018. PMID: 31458257 Free PMC article.
-
Langmuir monolayer of lysozyme at variable subphase pH conditions: a comprehensive study on structure, morphology and hysteresis behaviour.RSC Adv. 2023 Jul 27;13(33):22789-22799. doi: 10.1039/d3ra03710j. eCollection 2023 Jul 26. RSC Adv. 2023. PMID: 37520086 Free PMC article.
-
Unusual Aspects of Charge Regulation in Flexible Weak Polyelectrolytes.Polymers (Basel). 2023 Jun 14;15(12):2680. doi: 10.3390/polym15122680. Polymers (Basel). 2023. PMID: 37376324 Free PMC article. Review.
-
Increased preference for lysine over arginine in spike proteins of SARS-CoV-2 BA.2.86 variant and its daughter lineages.PLoS One. 2025 Apr 7;20(4):e0320891. doi: 10.1371/journal.pone.0320891. eCollection 2025. PLoS One. 2025. PMID: 40193474 Free PMC article.
References
-
- Leckband D., Israelachvili J. Intermolecular forces in biology. Q. Rev. Biophys. 2001;34:105–267. - PubMed
-
- Leckband D., Sivasankar S. Forces controlling protein interactions: theory and experiment. Colloids Surf. B Biointerfaces. 1999;14:83–97.
-
- Piazza R. Protein interactions and association: an open challenge for colloid science. Curr. Opin. Colloid Interface Sci. 2004;8:515–522.
-
- Simonson T. Electrostatics and dynamics of proteins. Rep. Prog. Phys. 2003;66:737–787.
-
- Woods L., Dalvit D., Podgornik R. A materials perspective on Casimir and van der Waals interactions. Rev. Mod. Phys. 2016;88:045003.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

