Omega-3 Fatty Acids Modulate TRPV4 Function through Plasma Membrane Remodeling
- PMID: 28978477
- PMCID: PMC8611619
- DOI: 10.1016/j.celrep.2017.09.029
Omega-3 Fatty Acids Modulate TRPV4 Function through Plasma Membrane Remodeling
Abstract
Dietary consumption of ω-3 polyunsaturated fatty acids (PUFAs), present in fish oils, is known to improve the vascular response, but their molecular targets remain largely unknown. Activation of the TRPV4 channel has been implicated in endothelium-dependent vasorelaxation. Here, we studied the contribution of ω-3 PUFAs to TRPV4 function by precisely manipulating the fatty acid content in Caenorhabditis elegans. By genetically depriving the worms of PUFAs, we determined that the metabolism of ω-3 fatty acids is required for TRPV4 activity. Functional, lipid metabolome, and biophysical analyses demonstrated that ω-3 PUFAs enhance TRPV4 function in human endothelial cells and support the hypothesis that lipid metabolism and membrane remodeling regulate cell reactivity. We propose a model whereby the eicosanoid's epoxide group location increases membrane fluidity and influences the endothelial cell response by increasing TRPV4 channel activity. ω-3 PUFA-like molecules might be viable antihypertensive agents for targeting TRPV4 to reduce systemic blood pressure.
Keywords: 17,18-epoxyeicosatetraenoic acid; Caenorhabditis elegans; TRP channels; TRPV4; atomic force microscopy; eicosapentaenoic acid; endothelial cells; fatty acids; neurons; polyunsaturated fatty acids.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no competing financial interests.
Figures



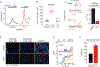
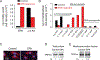


References
-
- Barden AE, Mas E, and Mori TA (2016). n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr Opin Lipidol 27, 26–32. - PubMed
-
- Bargmann CI (2006). Chemosensation in C. elegans. In WormBook, Jorgensen E, ed. (The C. elegans Research Community, WormBook, , http://www.wormbook.org/.), pp. 1–29. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

