Desynchronizing Embryonic Cell Division Waves Reveals the Robustness of Xenopus laevis Development
- PMID: 28978482
- PMCID: PMC5679461
- DOI: 10.1016/j.celrep.2017.09.017
Desynchronizing Embryonic Cell Division Waves Reveals the Robustness of Xenopus laevis Development
Abstract
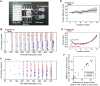
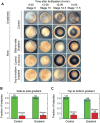
The early Xenopus laevis embryo is replete with dynamic spatial waves. One such wave, the cell division wave, emerges from the collective cell division timing of first tens and later hundreds of cells throughout the embryo. Here, we show that cell division waves do not propagate between neighboring cells and do not rely on cell-to-cell coupling to maintain their division timing. Instead, intrinsic variation in division period autonomously and gradually builds these striking patterns of cell division. Disrupting this pattern of division by placing embryos in a temperature gradient resulted in highly asynchronous entry to the midblastula transition and misexpression of the mesodermal marker Xbra. Remarkably, this gene expression defect is corrected during involution, resulting in delayed yet normal Xbra expression and viable embryos. This implies the existence of a previously unknown mechanism for normalizing mesodermal gene expression during involution.
Keywords: Xenopus laevis; asynchrony; cell cycle; cell division waves; early embryonic development; embryogenesis; mesoderm induction; metachronous cell division; midblastula transition; robustness.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signaling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. - PubMed
-
- Black SD. Experimental reversal of the normal dorsal-ventral timing of blastopore formation does not reverse axis polarity in Xenopus laevis embryos. Dev Biol. 1989;134:376–381. - PubMed
-
- Boterenbrood EC, Narraway JM, Hara K. Duration of cleavage cycles and asymmetry in the direction of cleavage waves prior to gastrulation in Xenopus laevis. Roux Arch Dev Biol. 1983;192:216–221. - PubMed
-
- Brown EE, Denegre JM, Danilchik MV. Deep cytoplasmic rearrangements in ventralized Xenopus embryos. Dev Biol. 1993;160:148–156. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

