Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13
- PMID: 28978555
- PMCID: PMC5627352
- DOI: 10.1136/bmj.j4530
Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13
Abstract
Objective To determine the availability of data on overall survival and quality of life benefits of cancer drugs approved in Europe.Design Retrospective cohort study.Setting Publicly accessible regulatory and scientific reports on cancer approvals by the European Medicines Agency (EMA) from 2009 to 2013.Main outcome measures Pivotal and postmarketing trials of cancer drugs according to their design features (randomisation, crossover, blinding), comparators, and endpoints. Availability and magnitude of benefit on overall survival or quality of life determined at time of approval and after market entry. Validated European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) used to assess the clinical value of the reported gains in published studies of cancer drugs.Results From 2009 to 2013, the EMA approved the use of 48 cancer drugs for 68 indications. Of these, eight indications (12%) were approved on the basis of a single arm study. At the time of market approval, there was significant prolongation of survival in 24 of the 68 (35%). The magnitude of the benefit on overall survival ranged from 1.0 to 5.8 months (median 2.7 months). At the time of market approval, there was an improvement in quality of life in seven of 68 indications (10%). Out of 44 indications for which there was no evidence of a survival gain at the time of market authorisation, in the subsequent postmarketing period there was evidence for extension of life in three (7%) and reported benefit on quality of life in five (11%). Of the 68 cancer indications with EMA approval, and with a median of 5.4 years' follow-up (minimum 3.3 years, maximum 8.1 years), only 35 (51%) had shown a significant improvement in survival or quality of life, while 33 (49%) remained uncertain. Of 23 indications associated with a survival benefit that could be scored with the ESMO-MCBS tool, the benefit was judged to be clinically meaningful in less than half (11/23, 48%).Conclusions This systematic evaluation of oncology approvals by the EMA in 2009-13 shows that most drugs entered the market without evidence of benefit on survival or quality of life. At a minimum of 3.3 years after market entry, there was still no conclusive evidence that these drugs either extended or improved life for most cancer indications. When there were survival gains over existing treatment options or placebo, they were often marginal.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure at www.icmje.org/coi_disclosure.pdf and declare: EG, AP, and EP were supported by project funding from Health Action International; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
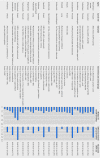
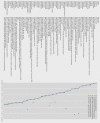
Comment in
-
Do cancer drugs improve survival or quality of life?BMJ. 2017 Oct 4;359:j4528. doi: 10.1136/bmj.j4528. BMJ. 2017. PMID: 28978548 Free PMC article.
-
Gravierende Zweifel an teuren Krebsmitteln.MMW Fortschr Med. 2017 Nov;159(19):44. doi: 10.1007/s15006-017-0272-4. MMW Fortschr Med. 2017. PMID: 29124588 Review. German. No abstract available.
-
Re: Availability of Evidence of Benefits on Overall Survival and Quality of Life of Cancer Drugs Approved by European Medicines Agency: Retrospective Cohort Study of Drug Approvals 2009-2013.Eur Urol. 2018 Apr;73(4):635-636. doi: 10.1016/j.eururo.2017.11.025. Epub 2017 Dec 6. Eur Urol. 2018. PMID: 29223606 No abstract available.
-
Demand cancer drugs that truly help patients.Nature. 2018 Apr;556(7700):151. doi: 10.1038/d41586-018-04154-9. Nature. 2018. PMID: 29636577 No abstract available.
References
-
- Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol 2011;29:755-60. 10.1200/JCO.2010.33.1744. pmid:21263086. - DOI - PubMed
-
- Tannock IF, Amir E, Booth CM, et al. Relevance of randomised controlled trials in oncology. Lancet Oncol 2016;17:e560-7. 10.1016/S1470-2045(16)30572-1. pmid:27924754. - DOI - PubMed
-
- Wilson MK, Collyar D, Chingos DT, et al. Outcomes and endpoints in cancer trials: bridging the divide. Lancet Oncol 2015;16:e43-52. 10.1016/S1470-2045(14)70380-8. pmid:25638556. - DOI - PubMed
-
- Amir E, Seruga B, Kwong R, Tannock IF, Ocaña A. Poor correlation between progression-free and overall survival in modern clinical trials: are composite endpoints the answer?Eur J Cancer 2012;48:385-8. 10.1016/j.ejca.2011.10.028. pmid:22115991. - DOI - PubMed
-
- Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol 2015;33:1008-14. 10.1200/JCO.2014.59.0489. pmid:25667291. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
