Calcineurin-mediated Dephosphorylation of Acetyl-coA Carboxylase is Required for Pheromone Biosynthesis Activating Neuropeptide (PBAN)-induced Sex Pheromone Biosynthesis in Helicoverpa armigera
- PMID: 28978618
- PMCID: PMC5724177
- DOI: 10.1074/mcp.RA117.000065
Calcineurin-mediated Dephosphorylation of Acetyl-coA Carboxylase is Required for Pheromone Biosynthesis Activating Neuropeptide (PBAN)-induced Sex Pheromone Biosynthesis in Helicoverpa armigera
Abstract
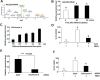
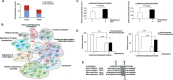
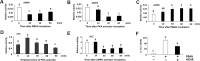
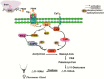
Chemical signaling plays a critical role in the behavior and physiology of many animals. Female insects, as many other animals, release sex pheromones to attract males for mating. The evolutionary and ecological success of insects therefore hinges on their ability to precisely mediate (including initiation and termination) pheromone biosynthesis. Pheromone biosynthesis activating neuropeptide (PBAN) acts directly on pheromone glands to regulate sex pheromone production using Ca2+ and cyclic-AMP as secondary messengers in the majority of species. However, the molecular mechanism downstream of the secondary messengers has not yet been elucidated in heliothine species. The present study shows that calcineurin, protein kinase A (PKA) and acetyl-coA carboxylase (ACC) are key components involved in PBAN-induced sex pheromone biosynthesis in Helicoverpa armigera using PBAN-dependent phosphoproteomics in combination with transcriptomics. RNAi-mediated knockdown and inhibitor assay demonstrated that calcineurin A is required for PBAN-induced ACC activation and sex pheromone production. Calcineurin-dependent phosphoproteomics and in vitro calcineurin phosphorylation assay further revealed that calcineurin regulated ACC activity by dephosphorylating ser84 and ser92. In addition, PKA-dependent phosphoproteomics and activity analysis revealed that PKA reduces the activity of AMP-activated protein kinase (AMPK), a negative regulator of ACC by phosphorylating the conserved ser92. Taken together, our findings indicate that calcineurin acts as the downstream signal of PBAN/G-protein receptor/Ca2+ to activate ACC through dephosphorylation while inactivating AMPK via PKA to reduce ACC phosphorylation, thus facilitating calcineurin activation of ACC.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
We declare no conflicts of interest
Figures




Similar articles
-
PBAN regulates sex pheromone biosynthesis by Ca2+/CaN/ACC and Ca2+/PKC/HK2 signal pathways in Spodoptera litura.Insect Mol Biol. 2025 Apr;34(2):347-362. doi: 10.1111/imb.12976. Epub 2024 Nov 16. Insect Mol Biol. 2025. PMID: 39548954
-
PKC phospho-activated PFK1 is required for PBAN regulated sex pheromone biosynthesis in Helicoverpa armigera.J Insect Physiol. 2025 Jan;160:104739. doi: 10.1016/j.jinsphys.2024.104739. Epub 2024 Dec 12. J Insect Physiol. 2025. PMID: 39674363
-
Calcineurin is required for male sex pheromone biosynthesis and female acceptance.Insect Mol Biol. 2018 Jun;27(3):373-382. doi: 10.1111/imb.12379. Epub 2018 Feb 21. Insect Mol Biol. 2018. PMID: 29465818
-
Pheromone biosynthesis activating neuropeptide (PBAN): regulatory role and mode of action.Gen Comp Endocrinol. 2009 May 15;162(1):69-78. doi: 10.1016/j.ygcen.2008.04.004. Epub 2008 Apr 18. Gen Comp Endocrinol. 2009. PMID: 18495120 Review.
-
Regulation of pheromone biosynthesis in moths.Curr Opin Insect Sci. 2017 Dec;24:29-35. doi: 10.1016/j.cois.2017.09.002. Epub 2017 Sep 14. Curr Opin Insect Sci. 2017. PMID: 29208220 Review.
Cited by
-
Quantitative analysis of protein crotonylation identifies its association with immunoglobulin A nephropathy.Mol Med Rep. 2020 Mar;21(3):1242-1250. doi: 10.3892/mmr.2020.10931. Epub 2020 Jan 13. Mol Med Rep. 2020. PMID: 32016442 Free PMC article.
-
Hexokinase Is Required for Sex Pheromone Biosynthesis in Helicoverpa armigera.Insects. 2021 Sep 30;12(10):889. doi: 10.3390/insects12100889. Insects. 2021. PMID: 34680657 Free PMC article.
-
Transcriptome-Wide Identification of Neuropeptides and Neuropeptide Receptors in the Twenty-Eight-Spotted Ladybird Henosepilachna vigintioctopunctata.Insects. 2025 Jun 13;16(6):624. doi: 10.3390/insects16060624. Insects. 2025. PMID: 40559054 Free PMC article.
-
Cyclosporin A as a Source for a Novel Insecticidal Product for Controlling Spodoptera frugiperda.Toxins (Basel). 2022 Oct 21;14(10):721. doi: 10.3390/toxins14100721. Toxins (Basel). 2022. PMID: 36287989 Free PMC article.
-
Transcriptomic Responses to Different Cry1Ac Selection Stresses in Helicoverpa armigera.Front Physiol. 2018 Nov 22;9:1653. doi: 10.3389/fphys.2018.01653. eCollection 2018. Front Physiol. 2018. PMID: 30524311 Free PMC article.
References
-
- Rafaeli A. (2002) Neuroendocrine control of pheromone biosynthesis in moths. Int. Rev. Cytol. 213, 49–91 - PubMed
-
- Engelmann F. (1960) Mechanisms controlling reproduction in two viviparous cockroaches (Blattaria). Ann. N.Y. Acad. Sci. 89, 516–536
-
- Riddiford L. M., and Williams C. M. (1971) Role of corpora cardiaca in the behavior of saturniid moths I. Release of sex pheromone. Biol. Bull. 140, 1–7 - PubMed
-
- Raina A. K., and Klun J. A. (1984) Brain factor control of sex pheromone production in the female corn earworm moth. Science 225, 531–533 - PubMed
-
- Raina A. K., Jaffe H., Kempe T. G., Keim P., Blacher R. W., Fales H. M. C., Riley T., Klun J. A., Ridgway R. L., and Hayes D. K. (1989) Identification of an neuropeptide hormone that regulates sex pheromone production in female moths. Science 244, 796–798 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

