Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials
- PMID: 28978660
- PMCID: PMC5664299
- DOI: 10.1212/WNL.0000000000004606
Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials
Erratum in
-
Correction: Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials.Neurology. 2017 Nov 28;89(22):2303. doi: 10.1212/WNL.0000000000004727. Neurology. 2017. PMID: 29180580 No abstract available.
Abstract
Objective: To assess whether genetic subgroups in recent amyotrophic lateral sclerosis (ALS) trials responded to treatment with lithium carbonate, but that the treatment effect was lost in a large cohort of nonresponders.
Methods: Individual participant data were obtained from 3 randomized trials investigating the efficacy of lithium carbonate. We matched clinical data with data regarding the UNC13A and C9orf72 genotype. Our primary outcome was survival at 12 months. On an exploratory basis, we assessed whether the effect of lithium depended on the genotype.
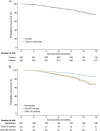
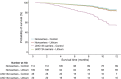
Results: Clinical data were available for 518 of the 606 participants. Overall, treatment with lithium carbonate did not improve 12-month survival (hazard ratio [HR] 1.0, 95% confidence interval [CI] 0.7-1.4; p = 0.96). Both the UNC13A and C9orf72 genotype were independent predictors of survival (HR 2.4, 95% CI 1.3-4.3; p = 0.006 and HR 2.5, 95% CI 1.1-5.2; p = 0.032, respectively). The effect of lithium was different for UNC13A carriers (p = 0.027), but not for C9orf72 carriers (p = 0.22). The 12-month survival probability for UNC13A carriers treated with lithium carbonate improved from 40.1% (95% CI 23.2-69.1) to 69.7% (95% CI 50.4-96.3).
Conclusions: This study incorporated genetic data into past ALS trials to determine treatment effects in a genetic post hoc analysis. Our results suggest that we should reorient our strategies toward finding treatments for ALS, start focusing on genotype-targeted treatments, and standardize genotyping in order to optimize randomization and analysis for future clinical trials.
Copyright © 2017 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
The beginning of precision medicine in ALS? Treatment to fit the genes.Neurology. 2017 Oct 31;89(18):1850-1851. doi: 10.1212/WNL.0000000000004612. Epub 2017 Oct 4. Neurology. 2017. PMID: 28978653 No abstract available.
References
-
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis: ALS/Riluzole Study Group. N Engl J Med 1994;330:585–591. - PubMed
-
- Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol 2014;13:1127–1138. - PubMed
-
- Su XW, Broach JR, Connor JR, Gerhard GS, Simmons Z. Genetic heterogeneity of amyotrophic lateral sclerosis: implications for clinical practice and research. Muscle Nerve 2014;49:786–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_G1000733/MRC_/Medical Research Council/United Kingdom
- MR/L501529/1/MRC_/Medical Research Council/United Kingdom
- G0600974/MRC_/Medical Research Council/United Kingdom
- TURNER/JAN13/944-795/MNDA_/Motor Neurone Disease Association/United Kingdom
- AL-CHALABI/APR15/844-791/MNDA_/Motor Neurone Disease Association/United Kingdom
- SHAW/APR15/933-794/MNDA_/Motor Neurone Disease Association/United Kingdom
- SHAW/NOV14/985-797/MNDA_/Motor Neurone Disease Association/United Kingdom
- JONES/OCT15/958-799/MNDA_/Motor Neurone Disease Association/United Kingdom
- G0300329/MRC_/Medical Research Council/United Kingdom
- G0900635/MRC_/Medical Research Council/United Kingdom
- MR/K01014X/1/MRC_/Medical Research Council/United Kingdom
- ALCHALABI-DOBSON/APR14/829-791/MNDA_/Motor Neurone Disease Association/United Kingdom
- MR/L021803/1/MRC_/Medical Research Council/United Kingdom
- G1100695/MRC_/Medical Research Council/United Kingdom
- G0501573/MRC_/Medical Research Council/United Kingdom
- G0500289/MRC_/Medical Research Council/United Kingdom
- ALCHALABI-TALBOT/APR14/926-794/MNDA_/Motor Neurone Disease Association/United Kingdom
- G0900688/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
