Rab27-Dependent Exosome Production Inhibits Chronic Inflammation and Enables Acute Responses to Inflammatory Stimuli
- PMID: 28978688
- PMCID: PMC5821227
- DOI: 10.4049/jimmunol.1700904
Rab27-Dependent Exosome Production Inhibits Chronic Inflammation and Enables Acute Responses to Inflammatory Stimuli
Abstract
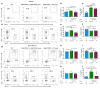
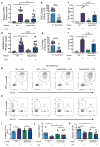
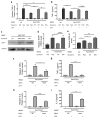
Extracellular vesicles, including exosomes, have recently been implicated as novel mediators of immune cell communication in mammals. However, roles for endogenously produced exosomes in regulating immune cell functions in vivo are just beginning to be identified. In this article, we demonstrate that Rab27a and Rab27b double-knockout (Rab27DKO) mice that are deficient in exosome secretion have a chronic, low-grade inflammatory phenotype characterized by elevated inflammatory cytokines and myeloproliferation. Upon further investigation, we found that some of these phenotypes could be complemented by wild-type (WT) hematopoietic cells or administration of exosomes produced by GM-CSF-expanded bone marrow cells. In addition, chronically inflamed Rab27DKO mice had a blunted response to bacterial LPS, resembling endotoxin tolerance. This defect was rescued by bone marrow exosomes from WT, but not miR-155-/-, cells, suggesting that uptake of miR-155-containing exosomes is important for a proper LPS response. Further, we found that SHIP1 and IRAK-M, direct targets of miR-155 that are known negative regulators of the LPS response, were elevated in Rab27DKO mice and decreased after treatment with WT, but not miR-155-/-, exosomes. Together, our study finds that Rab27-dependent exosome production contributes to homeostasis within the hematopoietic system and appropriate responsiveness to inflammatory stimuli.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

