Full-Length Isoforms of Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Accumulate in the Cytoplasm of Cells Undergoing the Lytic Cycle of Replication
- PMID: 28978712
- PMCID: PMC5709576
- DOI: 10.1128/JVI.01532-17
Full-Length Isoforms of Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Accumulate in the Cytoplasm of Cells Undergoing the Lytic Cycle of Replication
Abstract
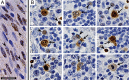
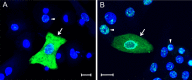
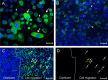
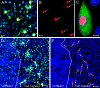
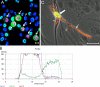
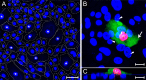
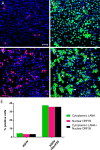
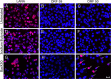
The latency-associated nuclear antigen (LANA) of the Kaposi's sarcoma-associated herpesvirus (KSHV) performs a variety of functions to establish and maintain KSHV latency. During latency, LANA localizes to discrete punctate spots in the nucleus, where it tethers viral episomes to cellular chromatin and interacts with nuclear components to regulate cellular and viral gene expression. Using highly sensitive tyramide signal amplification, we determined that LANA localizes to the cytoplasm in different cell types undergoing the lytic cycle of replication after de novo primary infection and after spontaneous, tetradecanoyl phorbol acetate-, or open reading frame 50 (ORF50)/replication transactivator (RTA)-induced activation. We confirmed the presence of cytoplasmic LANA in a subset of cells in lytically active multicentric Castleman disease lesions. The induction of cellular migration by scratch-wounding confluent cell cultures, culturing under subconfluent conditions, or induction of cell differentiation in primary cultures upregulated the number of cells permissive for primary lytic KSHV infection. The induction of lytic replication was characterized by high-level expression of cytoplasmic LANA and nuclear ORF59, a marker of lytic replication. Subcellular fractionation studies revealed the presence of multiple isoforms of LANA in the cytoplasm of ORF50/RTA-activated Vero cells undergoing primary infection. Mass spectrometry analysis demonstrated that cytoplasmic LANA isoforms were full length, containing the N-terminal nuclear localization signal. These results suggest that trafficking of LANA to different subcellular locations is a regulated phenomenon, which allows LANA to interact with cellular components in different compartments during both the latent and the replicative stages of the KSHV life cycle.IMPORTANCE Kaposi's sarcoma-associated herpesvirus (KSHV) causes AIDS-related malignancies, including lymphomas and Kaposi's sarcoma. KSHV establishes lifelong infections using its latency-associated nuclear antigen (LANA). During latency, LANA localizes to the nucleus, where it connects viral and cellular DNA complexes and regulates gene expression, allowing the virus to maintain long-term infections. Our research shows that intact LANA traffics to the cytoplasm of cells undergoing permissive lytic infections and latently infected cells in which the virus is induced to replicate. This suggests that LANA plays important roles in the cytoplasm and nuclear compartments of the cell during different stages of the KSHV life cycle. Determining cytoplasmic function and mechanism for regulation of the nuclear localization of LANA will enhance our understanding of the biology of this virus, leading to therapeutic approaches to eliminate infection and block its pathological effects.
Keywords: KSHV; LANA; activation; cytoplasm; mass spectrometry; migration; viral replication.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
-
IFI16 recruits HDAC1 and HDAC2 to deacetylate the Kaposi's sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen (LANA), facilitating latency.J Virol. 2025 Mar 18;99(3):e0154924. doi: 10.1128/jvi.01549-24. Epub 2025 Feb 10. J Virol. 2025. PMID: 39927772 Free PMC article.
-
Identification of Novel Kaposi's Sarcoma-Associated Herpesvirus Orf50 Transcripts: Discovery of New RTA Isoforms with Variable Transactivation Potential.J Virol. 2016 Dec 16;91(1):e01434-16. doi: 10.1128/JVI.01434-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795414 Free PMC article.
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
Control of Viral Latency by Episome Maintenance Proteins.Trends Microbiol. 2020 Feb;28(2):150-162. doi: 10.1016/j.tim.2019.09.002. Epub 2019 Oct 14. Trends Microbiol. 2020. PMID: 31624007 Free PMC article. Review.
-
Activation and counteraction of antiviral innate immunity by KSHV: an Update.Sci Bull (Beijing). 2018 Sep 30;63(18):1223-1234. doi: 10.1016/j.scib.2018.07.009. Epub 2018 Jul 20. Sci Bull (Beijing). 2018. PMID: 30906617 Free PMC article.
-
Lack of CD8+ T-cell co-localization with Kaposi's sarcoma-associated herpesvirus infected cells in Kaposi's sarcoma tumors.Oncotarget. 2020 Apr 28;11(17):1556-1572. doi: 10.18632/oncotarget.27569. eCollection 2020 Apr 28. Oncotarget. 2020. PMID: 32391124 Free PMC article.
-
Reduction of Kaposi's Sarcoma-Associated Herpesvirus Latency Using CRISPR-Cas9 To Edit the Latency-Associated Nuclear Antigen Gene.J Virol. 2019 Mar 21;93(7):e02183-18. doi: 10.1128/JVI.02183-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651362 Free PMC article.
-
Phase separation and DAXX redistribution contribute to LANA nuclear body and KSHV genome dynamics during latency and reactivation.PLoS Pathog. 2021 Jan 20;17(1):e1009231. doi: 10.1371/journal.ppat.1009231. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33471863 Free PMC article.
References
-
- Watanabe T, Sugaya M, Atkins AM, Aquilino EA, Yang A, Borris DL, Brady J, Blauvelt A. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J Virol 77:6188–6196. doi:10.1128/JVI.77.11.6188-6196.2003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

