Polarity sorting of axonal microtubules: a computational study
- PMID: 28978741
- PMCID: PMC5687029
- DOI: 10.1091/mbc.E17-06-0380
Polarity sorting of axonal microtubules: a computational study
Abstract
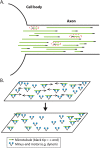
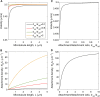
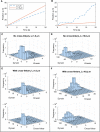
We present a computational model to test a "polarity sorting" mechanism for microtubule (MT) organization in developing axons. We simulate the motor-based axonal transport of short MTs to test the hypothesis that immobilized cytoplasmic dynein motors transport short MTs with their plus ends leading, so "mal-oriented" MTs with minus-end-out are transported toward the cell body while "correctly" oriented MTs are transported in the anterograde direction away from the soma. We find that dynein-based transport of short MTs can explain the predominately plus-end-out polarity pattern of axonal MTs but that transient attachments of plus-end-directed motor proteins and nonmotile cross-linker proteins are needed to explain the frequent pauses and occasional reversals observed in live-cell imaging of MT transport. Static cross-linkers increase the likelihood of a stalled "tug-of-war" between retrograde and anterograde forces on the MT, providing an explanation for the frequent pauses of short MTs and the immobility of longer MTs. We predict that inhibition of the proposed static cross-linker will produce disordered transport of short MTs and increased mobility of longer MTs. We also predict that acute inhibition of cytoplasmic dynein will disrupt the polarity sorting of MTs by increasing the likelihood of "incorrect" sorting of MTs by plus-end-directed motors.
© 2017 Craig et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures






Similar articles
-
Cytoplasmic Dynein Transports Axonal Microtubules in a Polarity-Sorting Manner.Cell Rep. 2017 Jun 13;19(11):2210-2219. doi: 10.1016/j.celrep.2017.05.064. Cell Rep. 2017. PMID: 28614709 Free PMC article.
-
Engineered Tug-of-War Between Kinesin and Dynein Controls Direction of Microtubule Based Transport In Vivo.Traffic. 2016 May;17(5):475-86. doi: 10.1111/tra.12385. Epub 2016 Mar 28. Traffic. 2016. PMID: 26843027 Free PMC article.
-
Regulation of Axonal Microtubule Polarity Orientation in Different Kinds of Neurons.FASEB J. 2025 Jun 15;39(11):e70683. doi: 10.1096/fj.202500675RR. FASEB J. 2025. PMID: 40458980
-
Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport.Neuron. 2017 Dec 20;96(6):1264-1271.e5. doi: 10.1016/j.neuron.2017.11.018. Epub 2017 Nov 30. Neuron. 2017. PMID: 29198755 Free PMC article. Review.
-
Microtubule-based transport along axons, dendrites and axonemes.Essays Biochem. 2000;35:89-102. doi: 10.1042/bse0350089. Essays Biochem. 2000. PMID: 12471892 Review.
Cited by
-
Bidirectional sliding of two parallel microtubules generated by multiple identical motors.J Math Biol. 2019 Jul;79(2):571-594. doi: 10.1007/s00285-019-01369-w. Epub 2019 Apr 23. J Math Biol. 2019. PMID: 31016335 Free PMC article.
-
An Integrated Cytoskeletal Model of Neurite Outgrowth.Front Cell Neurosci. 2018 Nov 26;12:447. doi: 10.3389/fncel.2018.00447. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30534055 Free PMC article. Review.
-
Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases.Int J Mol Sci. 2020 Oct 5;21(19):7354. doi: 10.3390/ijms21197354. Int J Mol Sci. 2020. PMID: 33027950 Free PMC article. Review.
-
Microtubule Organization Is Essential for Maintaining Cellular Morphology and Function.Oxid Med Cell Longev. 2022 Mar 7;2022:1623181. doi: 10.1155/2022/1623181. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35295719 Free PMC article. Review.
-
Mini-review: Microtubule sliding in neurons.Neurosci Lett. 2021 May 14;753:135867. doi: 10.1016/j.neulet.2021.135867. Epub 2021 Apr 1. Neurosci Lett. 2021. PMID: 33812935 Free PMC article. Review.
References
-
- Ahmad FJ, He Y, Myers KA, Hasaka TP, Francis F, Black MM, Baas PW. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. - PubMed
-
- Baas PW. Microtubule stability in the axon: new answers to an old mystery. Neuron. 2013;78:3–5. - PubMed
-
- Baas PW, Mozgova OI. A novel role for retrograde transport of microtubules in the axon. Cytoskeleton. 2012;69:416–425. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

