Convergence of linear acceleration and yaw rotation signals on non-eye movement neurons in the vestibular nucleus of macaques
- PMID: 28978765
- PMCID: PMC5866467
- DOI: 10.1152/jn.00382.2017
Convergence of linear acceleration and yaw rotation signals on non-eye movement neurons in the vestibular nucleus of macaques
Abstract
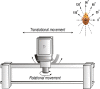
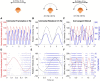
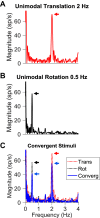
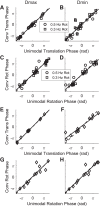
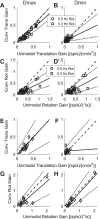
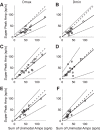
Roughly half of all vestibular nucleus neurons without eye movement sensitivity respond to both angular rotation and linear acceleration. Linear acceleration signals arise from otolith organs, and rotation signals arise from semicircular canals. In the vestibular nerve, these signals are carried by different afferents. Vestibular nucleus neurons represent the first point of convergence for these distinct sensory signals. This study systematically evaluated how rotational and translational signals interact in single neurons in the vestibular nuclei: multisensory integration at the first opportunity for convergence between these two independent vestibular sensory signals. Single-unit recordings were made from the vestibular nuclei of awake macaques during yaw rotation, translation in the horizontal plane, and combinations of rotation and translation at different frequencies. The overall response magnitude of the combined translation and rotation was generally less than the sum of the magnitudes in responses to the stimuli applied independently. However, we found that under conditions in which the peaks of the rotational and translational responses were coincident these signals were approximately additive. With presentation of rotation and translation at different frequencies, rotation was attenuated more than translation, regardless of which was at a higher frequency. These data suggest a nonlinear interaction between these two sensory modalities in the vestibular nuclei, in which coincident peak responses are proportionally stronger than other, off-peak interactions. These results are similar to those reported for other forms of multisensory integration, such as audio-visual integration in the superior colliculus. NEW & NOTEWORTHY This is the first study to systematically explore the interaction of rotational and translational signals in the vestibular nuclei through independent manipulation. The results of this study demonstrate nonlinear integration leading to maximum response amplitude when the timing and direction of peak rotational and translational responses are coincident.
Keywords: brain stem; multisensory integration; otolith; semicircular canal; single-unit recording.
Figures
Similar articles
-
Vestibular convergence patterns in vestibular nuclei neurons of alert primates.J Neurophysiol. 2002 Dec;88(6):3518-33. doi: 10.1152/jn.00518.2002. J Neurophysiol. 2002. PMID: 12466465
-
Encoding of head acceleration in vestibular neurons. I. Spatiotemporal response properties to linear acceleration.J Neurophysiol. 1993 Jun;69(6):2039-55. doi: 10.1152/jn.1993.69.6.2039. J Neurophysiol. 1993. PMID: 8350132
-
Gravity or translation: central processing of vestibular signals to detect motion or tilt.J Vestib Res. 2003;13(4-6):245-53. J Vestib Res. 2003. PMID: 15096668 Review.
-
Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: implication for perception.J Neurosci. 2015 Feb 25;35(8):3555-65. doi: 10.1523/JNEUROSCI.3540-14.2015. J Neurosci. 2015. PMID: 25716854 Free PMC article.
-
Otolith and canal integration on single vestibular neurons in cats.Exp Brain Res. 2005 Jul;164(3):271-85. doi: 10.1007/s00221-005-2341-7. Epub 2005 Jul 1. Exp Brain Res. 2005. PMID: 15991028 Review.
Cited by
-
Adaptation of spatio-temporal convergent properties in central vestibular neurons in monkeys.Physiol Rep. 2018 Sep;6(17):e13750. doi: 10.14814/phy2.13750. Physiol Rep. 2018. PMID: 30178612 Free PMC article.
-
Vestibular System and Self-Motion.Front Cell Neurosci. 2018 Nov 22;12:456. doi: 10.3389/fncel.2018.00456. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30524247 Free PMC article. Review.
-
Disrupted Rotational Perception During Simultaneous Stimulation of Rotation and Inertia.Cerebellum. 2024 Oct;23(5):2003-2011. doi: 10.1007/s12311-024-01698-7. Epub 2024 May 3. Cerebellum. 2024. PMID: 38702560
-
Temporal and spatial properties of vestibular signals for perception of self-motion.Front Neurol. 2023 Sep 13;14:1266513. doi: 10.3389/fneur.2023.1266513. eCollection 2023. Front Neurol. 2023. PMID: 37780704 Free PMC article. Review.
-
Transiently worse postural effects after vestibulo-ocular reflex gain-down adaptation in healthy adults.Exp Brain Res. 2024 Dec;242(12):2691-2699. doi: 10.1007/s00221-024-06923-7. Epub 2024 Oct 5. Exp Brain Res. 2024. PMID: 39368023
References
-
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

