Gene Tagging Strategies To Assess Protein Expression, Localization, and Function in Drosophila
- PMID: 28978772
- PMCID: PMC5629313
- DOI: 10.1534/genetics.117.199968
Gene Tagging Strategies To Assess Protein Expression, Localization, and Function in Drosophila
Erratum in
-
Gene Tagging Strategies To Assess Protein Expression, Localization, and Function in Drosophila.Genetics. 2017 Dec;207(4):1711. doi: 10.1534/genetics.117.300392. Genetics. 2017. PMID: 29203702 Free PMC article. No abstract available.
Abstract
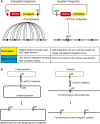
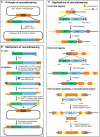
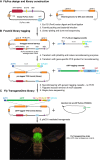
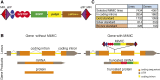
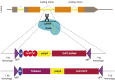
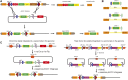
Analysis of gene function in complex organisms relies extensively on tools to detect the cellular and subcellular localization of gene products, especially proteins. Typically, immunostaining with antibodies provides these data. However, due to cost, time, and labor limitations, generating specific antibodies against all proteins of a complex organism is not feasible. Furthermore, antibodies do not enable live imaging studies of protein dynamics. Hence, tagging genes with standardized immunoepitopes or fluorescent tags that permit live imaging has become popular. Importantly, tagging genes present in large genomic clones or at their endogenous locus often reports proper expression, subcellular localization, and dynamics of the encoded protein. Moreover, these tagging approaches allow the generation of elegant protein removal strategies, standardization of visualization protocols, and permit protein interaction studies using mass spectrometry. Here, we summarize available genomic resources and techniques to tag genes and discuss relevant applications that are rarely, if at all, possible with antibodies.
Keywords: Drosophila; Flybook; gene tagging; genome engineering; techniques and resources; transgenesis.
Copyright © 2017 Kanca et al.
Figures



References
-
- Bassett A. R., Liu J.-L., 2014. CRISPR/Cas9 and genome editing in Drosophila. J. Genet. Genomics 41: 7–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

