Antiinflammatory effects of aprepitant coadministration with cART regimen containing ritonavir in HIV-infected adults
- PMID: 28978797
- PMCID: PMC5841871
- DOI: 10.1172/jci.insight.95893
Antiinflammatory effects of aprepitant coadministration with cART regimen containing ritonavir in HIV-infected adults
Abstract
Background: HIV-infected individuals, even well controlled with combined antiretroviral therapy (cART), have systemic inflammation and comorbidities. Substance P (SP) is an undecapeptide, which mediates neurotransmission and inflammation through its cognate neurokinin 1 receptor (NK1R). Plasma SP levels are elevated in HIV-infected individuals. The FDA-approved antiemetic aprepitant, an NK1R antagonist, has anti-HIV effects and antiinflammatory actions. We evaluated the safety, pharmacokinetics, and antiinflammatory properties of aprepitant in HIV-positive individuals receiving cART.
Methods: We conducted a phase 1B study of 12 HIV-positive individuals on a ritonavir-containing regimen (HIV viral load less than 40 copies/ml and CD4 > 400 cells/μl). Participants received open-label aprepitant 375 mg per day for 28 days and were followed for an additional 30 days. Changes in plasma levels of proinflammatory markers were assessed using flow cytometry, ELISA, luminex, and SOMAscan assays.
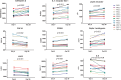
Results: The mean peak aprepitant plasma concentration was 30.7 ± 15.3 μg/ml at day 14 and 23.3 ± 12.3 μg/ml at day 28. Aprepitant treatment resulted in decreased plasma SP levels and affected 176 plasma proteins (56 after FDR) and several metabolic pathways, including inflammation and lipid metabolism. No change in soluble CD163 was observed. Aprepitant treatment was associated with a moderate increases in total and HDL cholesterol and affected select hematologic and metabolic markers, which returned to baseline levels 30 days after aprepitant treatment was stopped. There were 12 mild and 10 moderate adverse events (AE).
Conclusions: Aprepitant is safe and well tolerated. The antiinflammatory properties of aprepitant make it a possible adjunctive therapy for comorbid conditions associated with HIV infection.
Trial registration: ClinicalTrials.gov (NCT02154360).
Funding: This research was funded by NIH UO1 MH090325, P30 MH097488, and PO1 MH105303.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

