DOCK8 enforces immunological tolerance by promoting IL-2 signaling and immune synapse formation in Tregs
- PMID: 28978806
- PMCID: PMC5841868
- DOI: 10.1172/jci.insight.94298
DOCK8 enforces immunological tolerance by promoting IL-2 signaling and immune synapse formation in Tregs
Abstract
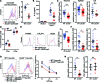
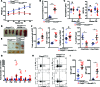
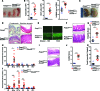
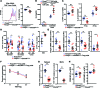
Patients deficient in the guanine nucleotide exchange factor DOCK8 have decreased numbers and impaired in vitro function of Tregs and make autoantibodies, but they seldom develop autoimmunity. We show that, similarly, Dock8-/- mice have decreased numbers and impaired in vitro function of Tregs but do not develop autoimmunity. In contrast, mice with selective DOCK8 deficiency in Tregs develop lymphoproliferation, autoantibodies, and gastrointestinal inflammation, despite a normal percentage and in vitro function of Tregs, suggesting that deficient T effector cell function might protect DOCK8-deficient patients from autoimmunity. We demonstrate that DOCK8 associates with STAT5 and is important for IL-2-driven STAT5 phosphorylation in Tregs. DOCK8 localizes within the lamellar actin ring of the Treg immune synapse (IS). Dock8-/- Tregs have abnormal TCR-driven actin dynamics, decreased adhesiveness, an altered gene expression profile, an unstable IS with decreased recruitment of signaling molecules, and impaired transendocytosis of the costimulatory molecule CD86. These data suggest that DOCK8 enforces immunological tolerance by promoting IL-2 signaling, TCR-driven actin dynamics, and the IS in Tregs.
Conflict of interest statement
Figures


References
-
- Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J Immunol. 1999;162(9):5078–5084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

