Functional role of PPAR-γ on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis
- PMID: 28978936
- PMCID: PMC5627284
- DOI: 10.1038/s41598-017-12570-6
Functional role of PPAR-γ on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis
Abstract
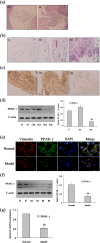
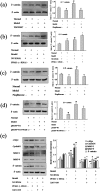
Peroxisome proliferator-activated receptor (PPAR)-γ is involved in both normal physiological processes and pathology of various diseases. The purpose of this study was to explore the function and underlying mechanisms of PPAR-γ in rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLSs) proliferation and migration. In the present study, we found PPAR-γ expression was remarkably reduced in RA synovium patient compare with OA and normal, as well as it was low-expression in Adjuvant-induced arthritis (AA). Moreover, inhibition PPAR-γ expression by T0070907 (12.5 μM) or PPAR-γ siRNA could promote FLSs proliferation and expressions of c-Myc, Cyclin D1, MMP-1, and MMP-9 in AA FLSs, except for TIPM-1. These date indicate that up-regulation of PPAR-γ may play a critical role in RA FLSs. Interestingly, co-incubation FLSs with Pioditazone (25 μM) and over expression vector with pEGFP-N1-PPAR-γ reduced proliferation and expressions of c-Myc, Cyclin D1, MMP-1, and MMP-9 in AA FLSs, besides TIMP-1. Further study indicates that PPAR-γ may induce activation Wnt/β-catenin signaling. In short, these results indicate that PPAR-γ may play a pivotal role during FLSs activation and activation of Wnt/β-catenin signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Dulos J, et al. p38 inhibition and not MK2 inhibition enhances the secretion of chemokines from TNF-alpha activated rheumatoid arthritis fibroblast-like synoviocytes. Clin Exp Rheumatol. 2013;31:515–525. - PubMed
-
- Koga T, et al. Post-transcriptional regulation of IL-6 production by Zc3h12a in fibroblast-like synovial cells. Clin Exp Rheumatol. 2011;29:906–912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

