Patient-reported outcome assessment of inflammatory arthritis patient experience with intravenously administered biologic therapy
- PMID: 28979103
- PMCID: PMC5602473
- DOI: 10.2147/PPA.S136567
Patient-reported outcome assessment of inflammatory arthritis patient experience with intravenously administered biologic therapy
Abstract
Objective: To evaluate patient perspectives regarding utilization of intravenous (IV) therapy for inflammatory arthritis (IA).
Methods: This was a single-center, noninterventional, patient questionnaire-based study of adult IA patients currently receiving IV biologics. At a single visit, patients completed the questionnaire comprising 30 questions centered on their experience receiving an intravenously administered therapy to treat their IA. The questionnaire included questions on patient demographics, disease characteristics, and previous biologic treatment for IA (subcutaneous [SC] and IV). Patients rated their level of agreement with statements regarding satisfaction with current IV biologic therapy and potential advantages and disadvantages of IV biologic therapy using a 5-point Likert scale (1= strongly disagree, 5= strongly agree).
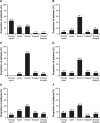
Results: One hundred patients were enrolled and completed the survey; 66% were female and the mean age was 58 years. Before IV treatment, 97% of patients received information regarding therapy options. Ninety patients ranked their satisfaction with current IV therapy as 4 or 5. The proportion of patients with an "extremely favorable" perception of IV therapy increased from 33% to 71% following initiation of their current medication. Thirty-one patients had previously received SC therapies to treat their IA.
Conclusion: These results demonstrated an overall favorable perception of IV therapy among this patient population. Patients previously treated with SC therapy also had a positive shift in the perception of IV therapy after initiating IV therapy. Patients' perception and preference for treatment options should be highly considered by the treating physician during or as part of a shared decision-making process.
Keywords: arthritis; biologic therapy; intravenous; patient satisfaction.
Conflict of interest statement
Disclosures NBG has received speaking fees from Janssen. WAK was an employee of Janssen Scientific Affairs, LLC, at the time this work was performed. SB, RD, and DP are employees of Janssen Scientific Affairs, LLC, and own stock in Johnson & Johnson, of which Janssen Scientific Affairs, LLC, is a wholly owned subsidiary. KLT was an employee of Janssen Research & Development, LLC, at the time this work was performed and owns stock in Johnson & Johnson, of which Janssen Research & Development, LLC, is a wholly owned subsidiary. The authors report no other conflicts of interest in this work.
Figures

References
-
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651–661. - PubMed
-
- Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study) Rheumatology (Oxford) 2010;49(2):289–294. - PubMed
-
- Salt E, Peden A. The complexity of the treatment: the decision-making process among women with rheumatoid arthritis. Qual Health Res. 2011;21(2):214–222. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

