Relevance of the Weber effect in contemporary pharmacovigilance of oncology drugs
- PMID: 28979130
- PMCID: PMC5602442
- DOI: 10.2147/TCRM.S137144
Relevance of the Weber effect in contemporary pharmacovigilance of oncology drugs
Abstract
Background: Numerous reporting biases have been known to affect spontaneous reporting databases. The Weber effect, which constitutes a peak in adverse event (AE) reporting of a drug at the end of second year after regulatory approval followed by a continuous decline thereafter, has been considered an important bias for a long time. The existence of this bias in AE reporting of oncology drugs remains an underevaluated area, prompting a targeted examination.
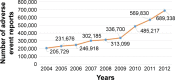
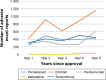
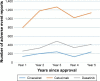
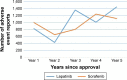
Methods: The US Food and Drug Administration (USFDA) Adverse Event Reporting System (FAERS) was studied for AE reporting patterns of 5 years of 15 new molecular entities (NMEs) and biologics used in oncology. This 5-year period started from the USFDA date of approval for the NMEs and biologics. The number of AEs reported for each of the drugs was plotted against time (years). The AE reporting patterns were specifically examined for the existence of the Weber effect. In addition, AE reporting rate patterns of 5 years of seven NMEs and biologics used in oncology were examined.
Results: A total of 50,630 AE reports were logged in to the FAERS for all 15 drugs examined for AE reporting patterns. We observed five distinct AE reporting patterns for 15 drugs; however, none of the AE patterns were identical to the Weber effect. We did not observe a consistent AE reporting rate pattern for the seven drugs examined for AE reporting rates. With the exception of one drug (cetuximab), none of the drugs exhibited a second-year peak in AE reporting rates. This peak was not followed by continuous decline in AE reporting rate thereafter.
Conclusion: This study does not support the existence of the Weber effect in AE reporting of oncology drugs. The contemporary AE reporting of oncology drugs does not exhibit a consistent pattern.
Keywords: FAERS; USFDA; Weber effect; adverse event reporting patterns; oncology drugs.
Conflict of interest statement
Disclosure RKJ and AA are employed by Sun Pharmaceutical Industries Limited. The authors report no conflicts of interest in this work.
Figures



References
-
- Weber JCP. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. In: Rainsford KD, Velo GD, editors. Side-effects of Anti-inflammatory Drugs, Advances in Inflammation Research. New York: Raven Press; 1984. pp. 1–7.
-
- Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004;24(6):743–749. - PubMed
-
- Hartnell NR, Wilson JP, Patel NC, Crismon ML. Adverse event reporting with selective serotonin-reuptake inhibitors. Ann Pharmacother. 2003;37(10):1387–1391. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

