Probiotic Mixture Golden Bifido Prevents Neonatal Escherichia coli K1 Translocation via Enhancing Intestinal Defense
- PMID: 28979247
- PMCID: PMC5611410
- DOI: 10.3389/fmicb.2017.01798
Probiotic Mixture Golden Bifido Prevents Neonatal Escherichia coli K1 Translocation via Enhancing Intestinal Defense
Abstract
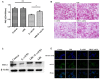
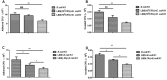
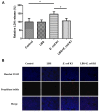
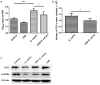
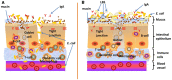
Escherichia coli (E. coli) K1 sepsis and meningitis is a severe infection characterized by high mortality in neonates. Successful colonization and translocation across the intestinal mucosa have been regarded as the critical steps for E. coli K1 sepsis and meningitis. We recently reported that the probiotic mixture, Golden Bifido (containing live Lactobacillus bulgaricus, Bifidobacterium, and Streptococcus thermophilus, LBS) has a preventive role against neonatal E. coli K1 bacteremia and meningitis. However, the interaction between the neonatal gut barrier, probiotics and E. coli K1 is still not elucidated. The present study aims to investigate how LBS exerts its protective effects on neonatal gut barrier during E. coli K1 infection. The beneficial effects of LBS were explored in vitro and in vivo using human colon carcinoma cell lines HT-29 and rat model of neonatal E. coli K1 infection, respectively. Our results showed that stimulation with E. coli K1 was able to cause intestinal barrier dysfunction, which were reflected by E. coli K1-induced intestinal damage and apoptosis of intestinal epithelial cells, reduction of mucin, immunoglobulin A (IgA) and tight junction proteins expression, as well as increase in intestinal permeability, all these changes facilitate E. coli K1 intestinal translocation. However, these changes were alleviated when HT-29 cells were treated with LBS before E. coli K1 infection. Furthermore, we found that LBS-treated neonatal rats (without E. coli K1 infection) have showed higher production of mucin, ZO-1, IgA, Ki67 in intestinal mucosa as well as lower intestinal permeability than that of non-treated rats, indicating that LBS could accelerate the development of neonatal intestinal defense. Taken together, our results suggest that enhancement of the neonatal intestinal defense to fight against E. coli K1 translocation could be the potential mechanism to elucidate how LBS confers a protective effect against neonatal E. coli K1 bacteremia and meningitis. This indirect mechanism makes LBS exert preventive effect on most of gut-derived pathogenic infections rather than only E. coli.
Keywords: bacterial translocation; intestinal barrier; mucin; neonatal sepsis and meningitis; probiotics; tight junction.
Figures


Similar articles
-
Mucin2 is Required for Probiotic Agents-Mediated Blocking Effects on Meningitic E. coli-Induced Pathogenicities.J Microbiol Biotechnol. 2015 Oct;25(10):1751-60. doi: 10.4014/jmb.1502.02010. J Microbiol Biotechnol. 2015. PMID: 26059517
-
Lactobacillus rhamnosus GG supernatant enhance neonatal resistance to systemic Escherichia coli K1 infection by accelerating development of intestinal defense.Sci Rep. 2017 Mar 6;7:43305. doi: 10.1038/srep43305. Sci Rep. 2017. PMID: 28262688 Free PMC article.
-
A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function.Front Microbiol. 2019 Mar 14;10:477. doi: 10.3389/fmicb.2019.00477. eCollection 2019. Front Microbiol. 2019. PMID: 30923519 Free PMC article.
-
Current concepts on Escherichia coli K1 translocation of the blood-brain barrier.FEMS Immunol Med Microbiol. 2004 Nov 1;42(3):271-9. doi: 10.1016/j.femsim.2004.09.001. FEMS Immunol Med Microbiol. 2004. PMID: 15477040 Review.
-
Neonatal meningitis due of Escherichia coli K1.J Infect Dis. 1977 Aug;136 Suppl:S93-7. doi: 10.1093/infdis/136.supplement.s93. J Infect Dis. 1977. PMID: 330780 Review.
Cited by
-
Revisiting the Intestinal Microbiome and Its Role in Diarrhea and Constipation.Microorganisms. 2023 Aug 29;11(9):2177. doi: 10.3390/microorganisms11092177. Microorganisms. 2023. PMID: 37764021 Free PMC article. Review.
-
Probiotic Cocktail Alleviates Intestinal Inflammation Through Improving Gut Microbiota and Metabolites in Colitis Mice.Front Cell Infect Microbiol. 2022 Jun 15;12:886061. doi: 10.3389/fcimb.2022.886061. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782138 Free PMC article.
-
[The postbiotic HM0539 from Lactobacillus rhamnosus GG prevents intestinal infection by enterohemorrhagic E. coli O157: H7 in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2020 Feb 29;40(2):211-218. doi: 10.12122/j.issn.1673-4254.2020.02.12. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32376527 Free PMC article. Chinese.
-
Application of Probiotic Yeasts on Candida Species Associated Infection.J Fungi (Basel). 2020 Sep 25;6(4):189. doi: 10.3390/jof6040189. J Fungi (Basel). 2020. PMID: 32992993 Free PMC article. Review.
-
Gut Microbiota and Diarrhea: An Updated Review.Front Cell Infect Microbiol. 2021 Apr 15;11:625210. doi: 10.3389/fcimb.2021.625210. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33937093 Free PMC article. Review.
References
-
- Bhagat R., Hussain S. Q., Gattoo I. A., Wani S. A. (2015). Incidence of meningitis in late onset sepsis. Int. J. Contemp. Pediatr. 2 96–102. 10.5455/2349-3291.ijcp20150507 - DOI
-
- Birchenough G. M., Johansson M. E., Stabler R. A., Dalgakiran F., Hansson G. C., Wren B. W., et al. (2013). Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect. Immun. 81 3264–3275. 10.1128/IAI.00268-13 - DOI - PMC - PubMed
-
- Blume C., David J., Bell R. E., Laver J. R., Read R. C., Clark G. C., et al. (2016). Modulation of human airway barrier functions during Burkholderia thailandensis and Francisella tularensis infection running title: airway barrier functions during bacterial infections. Pathogens 5:E53 10.3390/pathogens5030053 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

