TLR2-/- Mice Display Decreased Severity of Giardiasis via Enhanced Proinflammatory Cytokines Production Dependent on AKT Signal Pathway
- PMID: 28979269
- PMCID: PMC5611375
- DOI: 10.3389/fimmu.2017.01186
TLR2-/- Mice Display Decreased Severity of Giardiasis via Enhanced Proinflammatory Cytokines Production Dependent on AKT Signal Pathway
Abstract
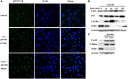
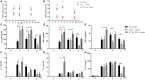
Giardia infection is one of the most common causes of waterborne diarrheal disease in a wide array of mammalian hosts, including humans globally. Although numerous studies have indicated that adaptive immune responses are important for Giardia defense, however, whether the host innate immune system such as TLRs recognizes Giardia remains poorly understood. TLR2 plays a crucial role in pathogen recognition, innate immunity activation, and the eventual pathogen elimination. In this study, we investigated the role of TLR2 as a non-protective inflammatory response on controlling the severity of giardiasis. RT-PCR analysis suggested that TLR2 expression was increased in vitro. We demonstrated that Giardia lamblia-induced cytokines expression by the activation of p38 and ERK pathways via TLR2. Interestingly, the expression of IL-12 p40, TNF-α, and IL-6, but not IFN-γ, was enhanced in TLR2-blocked and TLR2-/- mouse macrophages exposed to G. lamblia trophozoites compared with wild-type (WT) mouse macrophages. Further analysis demonstrated that G. lamblia trophozoites reduced cytokines secretion by activating AKT pathway in WT mouse macrophages. Immunohistochemical staining in G. lamblia cysts infected TLR2-/- and WT mice showed that TLR2 was highly expressed in duodenum in infected WT mice. Also, infected TLR2-/- and AKT-blocked mice showed an increased production of IL-12 p40 and IFN-γ compared with infected WT mice at the early stage during infection. Interestingly, infected TLR2-/- and AKT-blocked mice displayed a decreased parasite burden, an increased weight gain rate, and short parasite persistence. Histological morphometry showed shortened villus length, hyperplastic crypt and decreased ratio of villus height/crypt depth in infected WT mice compared with in infected TLR2-/- and AKT-blocked mice. Together, our results suggested that TLR2 deficiency leads to alleviation of giardiasis and reduction of parasite burden through the promotion of proinflammatory cytokines production. For the first time, our results demonstrated that TLR2 played a negative role in host defense against Giardia.
Keywords: AKT; Giardia; TLR2; TLR2−/−; cytokines; giardiasis.
Figures







Similar articles
-
Giardia duodenalis Induces Proinflammatory Cytokine Production in Mouse Macrophages via TLR9-Mediated p38 and ERK Signaling Pathways.Front Cell Dev Biol. 2021 Jul 15;9:694675. doi: 10.3389/fcell.2021.694675. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34336841 Free PMC article.
-
Mouse macrophages capture and kill Giardia lamblia by means of releasing extracellular trap.Dev Comp Immunol. 2018 Nov;88:206-212. doi: 10.1016/j.dci.2018.07.024. Epub 2018 Jul 24. Dev Comp Immunol. 2018. PMID: 30048699
-
Host defense against Neospora caninum infection via IL-12p40 production through TLR2/TLR3-AKT-ERK signaling pathway in C57BL/6 mice.Mol Immunol. 2021 Nov;139:140-152. doi: 10.1016/j.molimm.2021.08.019. Epub 2021 Sep 9. Mol Immunol. 2021. PMID: 34509754
-
Recent insights into the mucosal reactions associated with Giardia lamblia infections.Int J Parasitol. 2005 Nov;35(13):1339-47. doi: 10.1016/j.ijpara.2005.07.008. Epub 2005 Aug 24. Int J Parasitol. 2005. PMID: 16182298 Review.
-
Host defences against Giardia lamblia.Parasite Immunol. 2015 Aug;37(8):394-406. doi: 10.1111/pim.12210. Parasite Immunol. 2015. PMID: 26072999 Review.
Cited by
-
Extracellular Vesicles Secreted by Neospora caninum Are Recognized by Toll-Like Receptor 2 and Modulate Host Cell Innate Immunity Through the MAPK Signaling Pathway.Front Immunol. 2018 Jul 24;9:1633. doi: 10.3389/fimmu.2018.01633. eCollection 2018. Front Immunol. 2018. PMID: 30087675 Free PMC article.
-
Trichomonas vaginalis Induces Production of Proinflammatory Cytokines in Mouse Macrophages Through Activation of MAPK and NF-κB Pathways Partially Mediated by TLR2.Front Microbiol. 2018 Apr 10;9:712. doi: 10.3389/fmicb.2018.00712. eCollection 2018. Front Microbiol. 2018. PMID: 29692771 Free PMC article.
-
Giardia duodenalis and Its Secreted PPIB Trigger Inflammasome Activation and Pyroptosis in Macrophages through TLR4-Induced ROS Signaling and A20-Mediated NLRP3 Deubiquitination.Cells. 2021 Dec 6;10(12):3425. doi: 10.3390/cells10123425. Cells. 2021. PMID: 34943932 Free PMC article.
-
DDIT3 Targets Innate Immunity via the DDIT3-OTUD1-MAVS Pathway To Promote Bovine Viral Diarrhea Virus Replication.J Virol. 2021 Feb 24;95(6):e02351-20. doi: 10.1128/JVI.02351-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361422 Free PMC article.
-
Porcine Circovirus Type 2 Suppresses IL-12p40 Induction via Capsid/gC1qR-Mediated MicroRNAs and Signalings.J Immunol. 2018 Jul 15;201(2):533-547. doi: 10.4049/jimmunol.1800250. Epub 2018 Jun 1. J Immunol. 2018. PMID: 29858268 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

