Early detection of acute cisplatin nephrotoxicity: interest of urinary monitoring of proximal tubular biomarkers
- PMID: 28979774
- PMCID: PMC5622891
- DOI: 10.1093/ckj/sfx007
Early detection of acute cisplatin nephrotoxicity: interest of urinary monitoring of proximal tubular biomarkers
Abstract
Background: Renal toxicity induced by cisplatin (CisPt) is a clinical issue in patients with or without chronic kidney disease (CKD). Proximal tubular injury can result in acute kidney injury (AKI), which may compromise the course of chemotherapy and the prognosis. The purpose of this study was to investigate the time course of urinary markers of acute tubulotoxicity and to assess the usefulness of such monitoring in a routine clinical setting.
Methods: This work is an open prospective pilot study carried out among 23 patients receiving a platinum-based chemotherapy. Individual comorbidities, plasma parameters of kidney function (urea, creatinine) and estimated glomerular filtration rate were registered. Urinary excretion of leucine aminopeptidase, neutrophil gelatinase-associated lipocalin, cystatin C, liver fatty acid-binding protein and interleukin-18 were monitored during successive chemotherapy cycles. Episodes of AKI were identified according to KDIGO (Kidney Disease Improving Global Outcomes) 2012 guidelines.
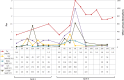
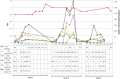
Results: A total of 28 patients were recruited; among them 23 agreed to be part of the study, of whom 18 received CisPt and 5 carbo- or oxaliplatin. Of the 18 CisPt patients, 12 had a preexisting CKD. Sixteen AKI episodes were observed in 13 patients receiving CisPt with a pejorative evolution in seven cases (partial recovery of the renal function); a transient but dramatic increase in urinary biomarkers was observed 3 h after chemotherapy initiation, whereas plasma creatinine rise appeared 72 h after the end of CisPt treatment. Identified precipitating factors included: dehydration due to lack of fluid intake or diuretic use, exposure to high CisPt doses, regular use of nonsteroidal anti-inflammatory drugs and/or iodinated contrast agents and sepsis.
Conclusion: Even if numerous precipitating factors could be avoided, the monitoring of urinary markers seemed helpful for the early detection of subclinical AKI induced during CisPt chemotherapy.
Keywords: AKI; biomarkers; cisplatin; creatinine; nephrotoxicity.
Figures
References
-
- Boulikas T, Vougiouka M.. Cisplatin and platinum drugs at the molecular level (Review). Oncol Rep 2003; 10: 1663–1682 - PubMed
-
- Filipski KK, Loos WJ, Verweij J. et al. Interaction of Cisplatin with the human organic cation transporter 2. Clin Cancer Res 2008; 14: 3875–3880 - PubMed
-
- Yokoo S, Yonezawa A, Masuda S. et al. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 2007; 74: 477–487 - PubMed
-
- dos Santos NA, Carvalho Rodrigues MA, Martins NM. et al. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 2012; 86: 1233–1250 - PubMed
-
- Yao X, Panichpisal K, Kurtzman N. et al. Cisplatin nephrotoxicity: a review. Am J Med Sci 2007; 334: 115–124 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources