MicroRNA-625 inhibits the proliferation and increases the chemosensitivity of glioma by directly targeting AKT2
- PMID: 28979807
- PMCID: PMC5622219
MicroRNA-625 inhibits the proliferation and increases the chemosensitivity of glioma by directly targeting AKT2
Abstract
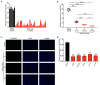
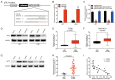
Glioma is a malignant tumor for which new therapies are needed. Growing evidence has demonstrated that microRNAs (miRNAs) have a major effect on glioma development. Here, we aimed to characterize a novel anti-cancer miRNA, miR-625, by investigating its expression, function, and mechanism of action in glioma progression. The expression of miR-625 and its target mRNA in human glioma tissues and cell lines was assessed by real-time PCR, western blotting, and immunohistochemistry. Functional significance was assessed by examining cell cycle progression, proliferation, apoptosis, and chemosensitivity to temozolomide in vitro, and by examining growth of subcutaneous glioblastoma in a mouse model in vivo. We found that miR-625 expression was significantly lower in human glioma samples and cell lines than in normal brain tissue and human astrocytes. Furthermore, miR-625 overexpression not only suppressed glioma cell proliferation in culture and in the tumor xenograft model but also induced cell cycle arrest and apoptosis. AKT2 was identified as a direct miR-625 target in glioma cell lines, and AKT2 overexpression reversed the suppressive effects of miR-625 in the cell lines and the tumor xenograft model. Finally, we found that the sensitivity of glioma cells to temozolomide was increased by miR-625 overexpression, and this was reversed by concomitant AKT2 expression. In conclusion, our findings suggest that the miR-625-AKT2 axis could be a new prognostic marker and diagnostic target for gliomas.
Keywords: AKT2; Glioma; miR-625; proliferation.
Conflict of interest statement
None.
Figures




Similar articles
-
miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas.Neuro Oncol. 2017 Jan;19(1):55-65. doi: 10.1093/neuonc/now129. Epub 2016 Jul 28. Neuro Oncol. 2017. PMID: 27471108 Free PMC article.
-
miRNA-193a-3p Regulates the AKT2 Pathway to Inhibit the Growth and Promote the Apoptosis of Glioma Cells by Targeting ALKBH5.Front Oncol. 2021 Apr 23;11:600451. doi: 10.3389/fonc.2021.600451. eCollection 2021. Front Oncol. 2021. Retraction in: Front Oncol. 2021 Dec 29;11:840650. doi: 10.3389/fonc.2021.840650. PMID: 33968717 Free PMC article. Retracted.
-
The miR-26a/AP-2α/Nanog signaling axis mediates stem cell self-renewal and temozolomide resistance in glioma.Theranostics. 2019 Jul 28;9(19):5497-5516. doi: 10.7150/thno.33800. eCollection 2019. Theranostics. 2019. PMID: 31534499 Free PMC article.
-
MicroRNA-1294 inhibits the proliferation and enhances the chemosensitivity of glioma to temozolomide via the direct targeting of TPX2.Am J Cancer Res. 2018 Feb 1;8(2):291-301. eCollection 2018. Am J Cancer Res. 2018. PMID: 29511599 Free PMC article.
-
miR-137: A Novel Therapeutic Target for Human Glioma.Mol Ther Nucleic Acids. 2020 Sep 4;21:614-622. doi: 10.1016/j.omtn.2020.06.028. Epub 2020 Jun 30. Mol Ther Nucleic Acids. 2020. PMID: 32736290 Free PMC article. Review.
Cited by
-
Hypoxia-associated circDENND2A promotes glioma aggressiveness by sponging miR-625-5p.Cell Mol Biol Lett. 2019 Apr 2;24:24. doi: 10.1186/s11658-019-0149-x. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 30988674 Free PMC article.
-
Selective regulation of chemosensitivity in glioblastoma by phosphatidylinositol 3-kinase beta.iScience. 2024 May 7;27(6):109921. doi: 10.1016/j.isci.2024.109921. eCollection 2024 Jun 21. iScience. 2024. PMID: 38812542 Free PMC article.
-
Crucial Roles of miR-625 in Human Cancer.Front Med (Lausanne). 2022 Mar 4;9:845094. doi: 10.3389/fmed.2022.845094. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35308517 Free PMC article. Review.
-
The Role of microRNAs in Multidrug Resistance of Glioblastoma.Cancers (Basel). 2022 Jun 30;14(13):3217. doi: 10.3390/cancers14133217. Cancers (Basel). 2022. PMID: 35804989 Free PMC article. Review.
-
MicroRNAs as the pivotal regulators of Temozolomide resistance in glioblastoma.Mol Brain. 2024 Jul 2;17(1):42. doi: 10.1186/s13041-024-01113-6. Mol Brain. 2024. PMID: 38956588 Free PMC article. Review.
References
-
- Linz U. Commentary on effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial (Lancet Oncol. 2009;10:459-466) Cancer. 2010;116:1844–1846. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
