Clinic-Based Urinary Lipoarabinomannan as a Biomarker of Clinical Disease Severity and Mortality Among Antiretroviral Therapy-Naive Human Immunodeficiency Virus-Infected Adults in South Africa
- PMID: 28979922
- PMCID: PMC5622366
- DOI: 10.1093/ofid/ofx167
Clinic-Based Urinary Lipoarabinomannan as a Biomarker of Clinical Disease Severity and Mortality Among Antiretroviral Therapy-Naive Human Immunodeficiency Virus-Infected Adults in South Africa
Abstract
Background: Urinary lipoarabinomannan (LAM) has limited sensitivity for diagnosing active human immunodeficiency virus (HIV)-associated tuberculosis (TB) disease, but LAM screening at HIV diagnosis might identify adults with more severe clinical disease or greater risk of mortality.
Methods: We enrolled antiretroviral therapy-naive HIV-infected adults from 4 clinics in Durban. Nurses performed urine LAM testing using a rapid assay (Determine TB LAM) graded from low (1+) to high (≥3+) intensity. Urine LAM results were not used to guide anti-TB therapy. We assessed TB-related symptoms and obtained sputum for mycobacterial smear and culture. Participants were observed for 12 months, and we used multivariable Cox proportional hazard models to determine hazard ratios for all-cause mortality.
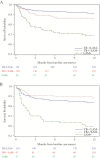
Results: Among 726 HIV-infected adults with median CD4 of 205 cells/mm3 (interquartile range, 79-350 cells/mm3), 93 (13%) were LAM positive and 89 (12%) participants died during the follow-up period. In multivariable analyses, urine LAM-positive participants had a mortality hazard ratio (MHR) of 3.58 (95% confidence interval [CI], 2.20-5.81) for all-cause mortality. Among participants with mycobacterial-confirmed TB, urine LAM-positivity had a 2.91 (95% CI, 1.26-6.73) MHR for all participants and a 4.55 (95% CI, 1.71-12.1) MHR for participants with CD4 ≤100 cell/mm3. Participants with LAM-positive TB had significantly more clinical signs and symptoms of disease, compared with participants with LAM-negative TB disease.
Conclusions: Among HIV-infected adults, urinary LAM-positive patients had more clinical disease severity and a 3-fold increase in 12-month mortality compared with those who were LAM negative.
Keywords: HIV/AIDS; South Africa; lipoarabinomannan (LAM); tuberculosis; urine.
Figures
References
-
- World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015.
-
- World Health Organization. WHO Policy on Collaborative TB/HIV Activities. Geneva: World Health Organization; 2012.
-
- World Health Organization, Stop TB. “End TB Strategy.” Geneva: World Health Organization; 2014.
-
- World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. Geneva: World Health Organization; 2013. - PubMed
-
- World Health Organization. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living With HIV in Resource-Constrained Settings. Geneva: World Health Organization; 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

