Therapeutic Effects of a Novel Agonist of Peroxisome Proliferator-Activated Receptor Alpha for the Treatment of Diabetic Retinopathy
- PMID: 28979999
- PMCID: PMC5633008
- DOI: 10.1167/iovs.16-21402
Therapeutic Effects of a Novel Agonist of Peroxisome Proliferator-Activated Receptor Alpha for the Treatment of Diabetic Retinopathy
Abstract
Purpose: Clinical studies have shown that peroxisome proliferator-activated receptor alpha (PPARα) agonist fenofibrate has therapeutic effects on diabetic retinopathy (DR). The purpose of this study was to identify a novel PPARα agonist and to evaluate its beneficial effects on DR.
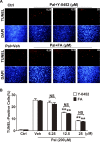
Methods: The transcriptional activity of PPARα was measured by a luciferase-based promoter assay. TUNEL was used to evaluate apoptosis in retinal precursor cells (R28). Diabetes was induced in rats by injection of streptozotocin. Retinal inflammation was examined using leukostasis assay, and retinal vascular leakage was measured using permeability assay. Retinal function was measured using electroretinogram (ERG) recording, and retinal apoptosis was quantified using the cell death ELISA. The anti-angiogenic effect was evaluated in the oxygen-induced retinopathy (OIR) model.
Results: A compound, 7-chloro-8-methyl-2-phenylquinoline-4-carboxylic acid (Y-0452), with a chemical structure distinct from existing PPARα agonists, activated PPARα transcriptional activity and upregulated PPARα expression. Y-0452 significantly inhibited human retinal capillary endothelial cell migration and tube formation. The compound also protected R28 cells against apoptosis and inhibited NF-κB signaling in R28 cells exposed to palmitate. In diabetic rats, Y-0452 ameliorated leukostasis and vascular leakage in the retina. In addition, Y-0452 preserved the retinal function and reduced retinal cell death in diabetic rats. Y-0452 also alleviated retinal neovascularization in the OIR model.
Conclusions: Y-0452 is a novel PPARα agonist and has therapeutic potential for DR.
Figures








References
-
- Fong DS, Sharza M, Chen W, Paschal JF, Ariyasu RG, Lee PP. . Vision loss among diabetics in a group model Health Maintenance Organization (HMO). Am J Ophthalmol. 2002; 133: 236– 241. - PubMed
-
- Xie TY, Yan W, Lou J, Chen XY. . Effect of ozone on vascular endothelial growth factor (VEGF) and related inflammatory cytokines in rats with diabetic retinopathy. Genet Mol Res. 2016; 15: 1– 11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

