ReMAPping the microtubule landscape: How phosphorylation dictates the activities of microtubule-associated proteins
- PMID: 28980356
- PMCID: PMC5739964
- DOI: 10.1002/dvdy.24599
ReMAPping the microtubule landscape: How phosphorylation dictates the activities of microtubule-associated proteins
Abstract
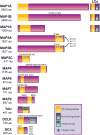
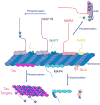
Classical microtubule-associated proteins (MAPs) were originally identified based on their co-purification with microtubules assembled from mammalian brain lysate. They have since been found to perform a range of functions involved in regulating the dynamics of the microtubule cytoskeleton. Most of these MAPs play integral roles in microtubule organization during neuronal development, microtubule remodeling during neuronal activity, and microtubule stabilization during neuronal maintenance. As a result, mutations in MAPs contribute to neurodevelopmental disorders, psychiatric conditions, and neurodegenerative diseases. MAPs are post-translationally regulated by phosphorylation depending on developmental time point and cellular context. Phosphorylation can affect the microtubule affinity, cellular localization, or overall function of a particular MAP and can thus have profound implications for neuronal health. Here we review MAP1, MAP2, MAP4, MAP6, MAP7, MAP9, tau, and DCX, and how each is regulated by phosphorylation in neuronal physiology and disease. Developmental Dynamics 247:138-155, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: MAPs; cytoskeleton; kinases; microtubules; neurodegeneration; neuronal development.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Ainsztein AM, Purich DL. Stimulation of tubulin polymerization by MAP-2. Control by protein kinase C-mediated phosphorylation at specific sites in the microtubule-binding region. J Biol Chem. 1994;269:28465–28471. - PubMed
-
- Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123:3415–3419. - PubMed
-
- Andersen SS, Wittmann T. Toward reconstitution of in vivo microtubule dynamics in vitro. Bioessays. 2002;24:305–307. - PubMed
-
- Andrieux A, Salin PA, Vernet M, Kujala P, Baratier J, Gory-Faure S, Bosc C, Pointu H, Proietto D, Schweitzer A, Denarier E, Klumperman J, Job D. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes Dev. 2002;16:2350–2364. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

