Enantioselective Rhodium-Catalyzed Coupling of Arylboronic Acids, 1,3-Enynes, and Imines by Alkenyl-to-Allyl 1,4-Rhodium(I) Migration
- PMID: 28980437
- PMCID: PMC5765452
- DOI: 10.1002/anie.201709334
Enantioselective Rhodium-Catalyzed Coupling of Arylboronic Acids, 1,3-Enynes, and Imines by Alkenyl-to-Allyl 1,4-Rhodium(I) Migration
Abstract
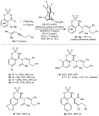
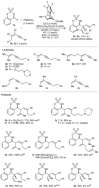
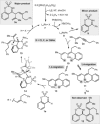
A chiral rhodium complex catalyzes the highly enantioselective coupling of arylboronic acids, 1,3-enynes, and imines to give homoallylic sulfamates. The key step is the generation of allylrhodium(I) species by alkenyl-to-allyl 1,4-rhodium(I) migration.
Keywords: allylation; asymmetric catalysis; imines; isomerization; rhodium.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The isomerization of allylrhodium intermediates in the rhodium-catalyzed nucleophilic allylation of cyclic imines.Angew Chem Int Ed Engl. 2014 Oct 20;53(43):11605-10. doi: 10.1002/anie.201407233. Epub 2014 Sep 9. Angew Chem Int Ed Engl. 2014. PMID: 25205604 Free PMC article.
-
Arylative Intramolecular Allylation of Ketones with 1,3-Enynes Enabled by Catalytic Alkenyl-to-Allyl 1,4-Rhodium(I) Migration.Angew Chem Int Ed Engl. 2017 Jun 12;56(25):7227-7232. doi: 10.1002/anie.201703155. Epub 2017 May 19. Angew Chem Int Ed Engl. 2017. PMID: 28523779 Free PMC article.
-
One-Carbon Oxidative Annulations of 1,3-Enynes by Catalytic C-H Functionalization and 1,4-Rhodium(III) Migration.Chemistry. 2018 Mar 15;24(16):4050-4054. doi: 10.1002/chem.201706043. Epub 2018 Feb 21. Chemistry. 2018. PMID: 29356188
-
Rhodium(III)-Catalyzed Asymmetric [4+1] and [5+1] Annulation of Arenes and 1,3-Enynes: A Distinct Mechanism of Allyl Formation and Allyl Functionalization.Angew Chem Int Ed Engl. 2020 Dec 7;59(50):22706-22713. doi: 10.1002/anie.202010832. Epub 2020 Oct 7. Angew Chem Int Ed Engl. 2020. PMID: 32886841
-
Application of Rh(I)/Bicyclo[2.2.1]heptadiene Catalysts to the Enantioselective Synthesis of Chiral Amines.Chem Rec. 2021 Dec;21(12):3954-3963. doi: 10.1002/tcr.202100209. Epub 2021 Oct 1. Chem Rec. 2021. PMID: 34596958 Review.
Cited by
-
Rhodium(iii)-catalyzed diverse [4 + 1] annulation of arenes with 1,3-enynes via sp3/sp2 C-H activation and 1,4-rhodium migration.Chem Sci. 2019 Feb 26;10(14):3987-3993. doi: 10.1039/c9sc00545e. eCollection 2019 Apr 14. Chem Sci. 2019. PMID: 31015939 Free PMC article.
-
Enantio- and Diastereoselective Synthesis of Homopropargyl Amines by Copper-Catalyzed Coupling of Imines, 1,3-Enynes, and Diborons.Angew Chem Int Ed Engl. 2020 Mar 16;59(12):4879-4882. doi: 10.1002/anie.201915191. Epub 2020 Feb 11. Angew Chem Int Ed Engl. 2020. PMID: 31917893 Free PMC article.
-
Catalytic enantioselective arylative cyclizations of alkynyl 1,3-diketones by 1,4-rhodium(i) migration.Chem Sci. 2020 Feb 6;11(10):2759-2764. doi: 10.1039/c9sc06309a. Chem Sci. 2020. PMID: 34084335 Free PMC article.
References
-
- For recent reviews, see:
-
- Huo H.-X., Duvall J. R., Huang M.-Y., Hong R., Org. Chem. Front. 2014, 1, 303–320;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

