The roles of intrinsic disorder-based liquid-liquid phase transitions in the "Dr. Jekyll-Mr. Hyde" behavior of proteins involved in amyotrophic lateral sclerosis and frontotemporal lobar degeneration
- PMID: 28980860
- PMCID: PMC5788550
- DOI: 10.1080/15548627.2017.1384889
The roles of intrinsic disorder-based liquid-liquid phase transitions in the "Dr. Jekyll-Mr. Hyde" behavior of proteins involved in amyotrophic lateral sclerosis and frontotemporal lobar degeneration
Abstract
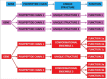
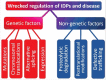
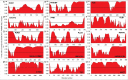
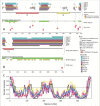
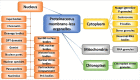
Pathological developments leading to amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) are associated with misbehavior of several key proteins, such as SOD1 (superoxide dismutase 1), TARDBP/TDP-43, FUS, C9orf72, and dipeptide repeat proteins generated as a result of the translation of the intronic hexanucleotide expansions in the C9orf72 gene, PFN1 (profilin 1), GLE1 (GLE1, RNA export mediator), PURA (purine rich element binding protein A), FLCN (folliculin), RBM45 (RNA binding motif protein 45), SS18L1/CREST, HNRNPA1 (heterogeneous nuclear ribonucleoprotein A1), HNRNPA2B1 (heterogeneous nuclear ribonucleoprotein A2/B1), ATXN2 (ataxin 2), MAPT (microtubule associated protein tau), and TIA1 (TIA1 cytotoxic granule associated RNA binding protein). Although these proteins are structurally and functionally different and have rather different pathological functions, they all possess some levels of intrinsic disorder and are either directly engaged in or are at least related to the physiological liquid-liquid phase transitions (LLPTs) leading to the formation of various proteinaceous membrane-less organelles (PMLOs), both normal and pathological. This review describes the normal and pathological functions of these ALS- and FTLD-related proteins, describes their major structural properties, glances at their intrinsic disorder status, and analyzes the involvement of these proteins in the formation of normal and pathological PMLOs, with the ultimate goal of better understanding the roles of LLPTs and intrinsic disorder in the "Dr. Jekyll-Mr. Hyde" behavior of those proteins.
Keywords: amyotrophic lateral sclerosis; frontotemporal lobar degeneration; intrinsically disordered proteins; liquid-liquid phase transition; neurodegeneration; proteinaceous membrane-less organelles.
Figures

References
-
- Kim HJ, de Leon M, Wang X, Kim HY, Lee YJ, Kim YH, Kim SH. Relationship between clinical parameters and brain structure in sporadic amyotrophic lateral sclerosis patients according to onset type: A voxel-based morphometric study. PLoS One. 2017;12:e0168424. doi: 10.1371/journal.pone.0168424. PMID:28095425. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
